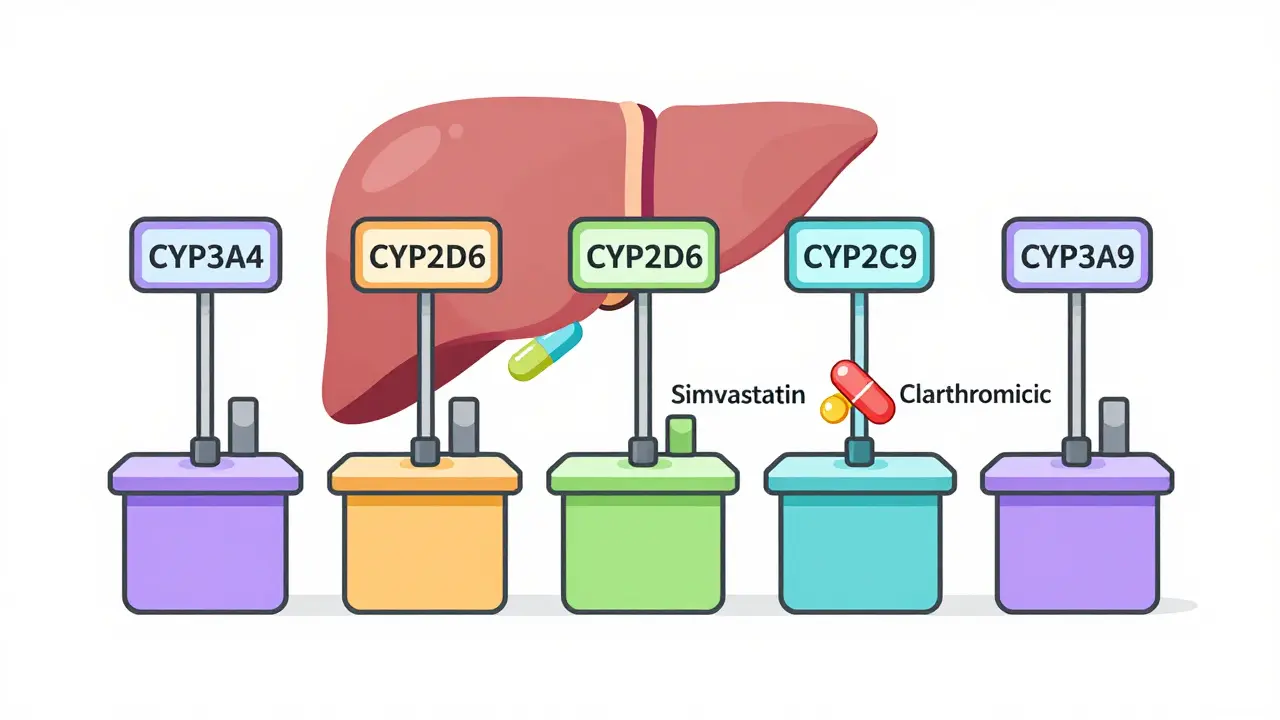
Fertility Preservation Before Chemotherapy: Options, Success Rates, and Timing
- Dorian Wakefield
- 4 Feb 2026
- Medicine
Fertility preservation before chemotherapy helps cancer patients protect their ability to have children. Learn about egg freezing, sperm banking, ovarian tissue options, success rates, timing, and insurance. Real patient stories and expert advice.
View More