Chronic Hepatitis C: How Modern Antivirals Cure the Virus and Protect the Liver
- Dorian Wakefield
- 3 Feb 2026
- Medicine
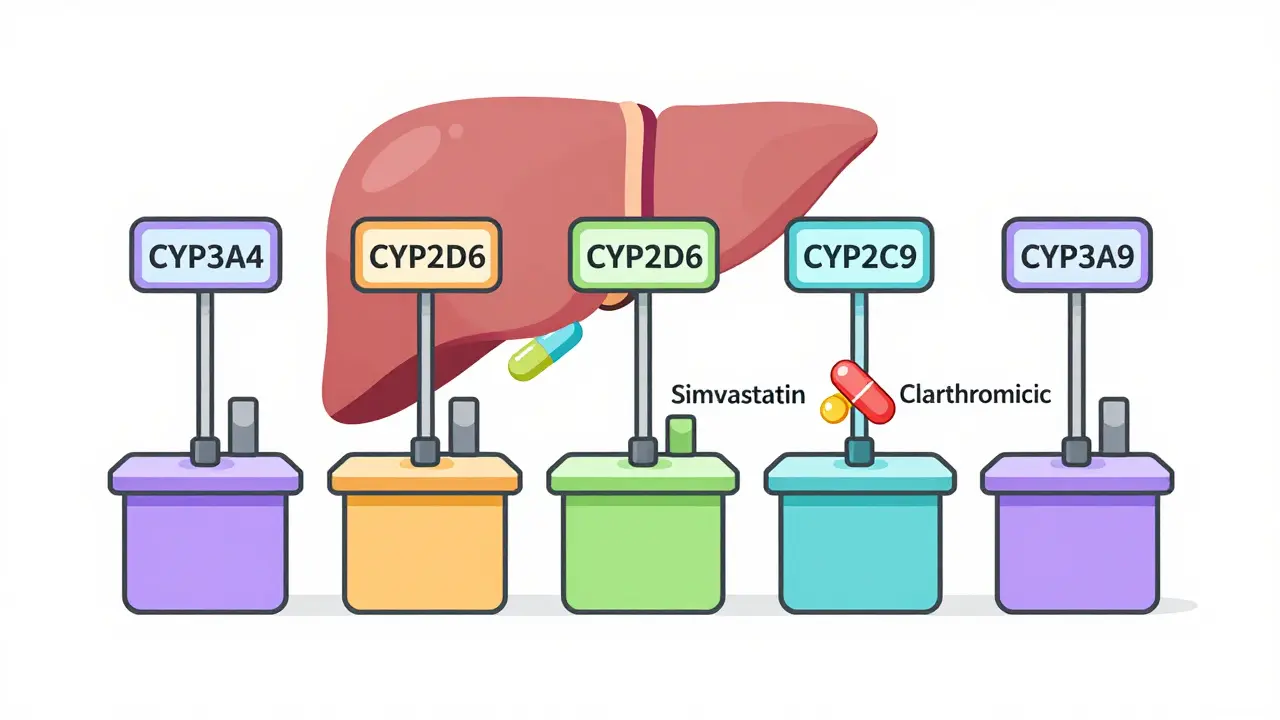
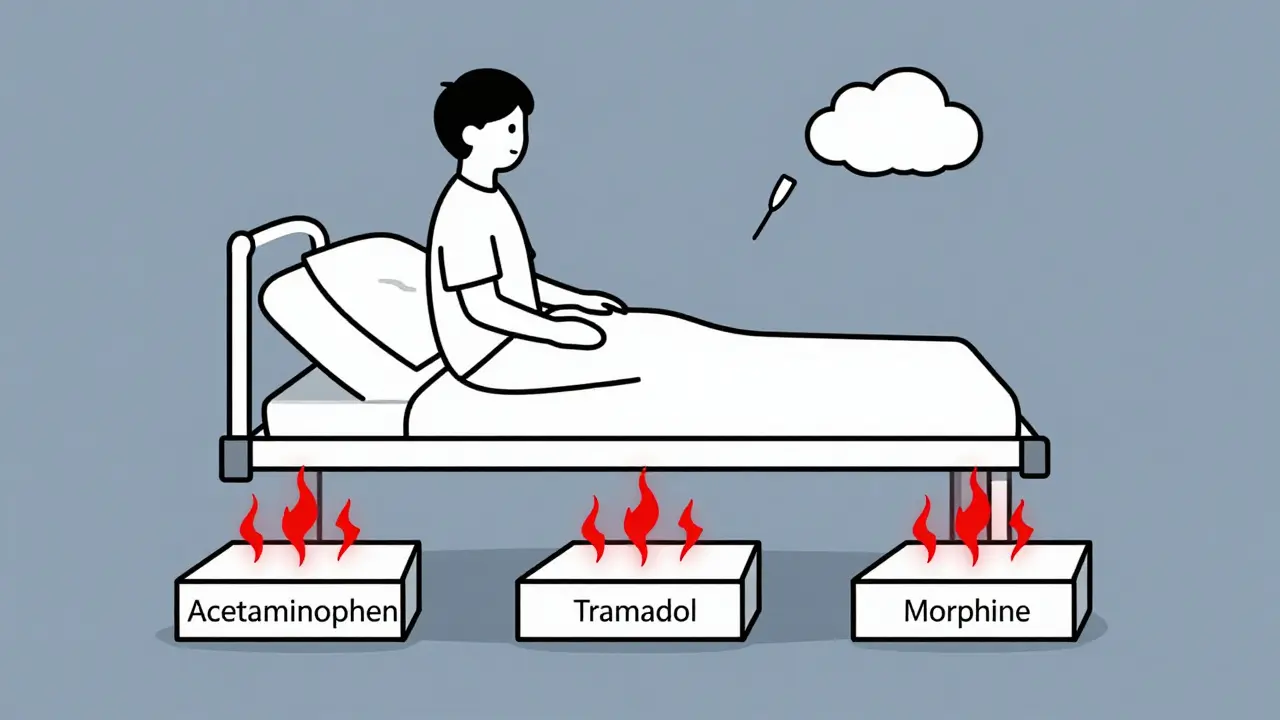
Chronic hepatitis C is now curable with simple oral antivirals that achieve over 95% success rates. These modern treatments stop liver damage, allow healing, and eliminate the need for harsh therapies. Access and testing remain key barriers.
View More