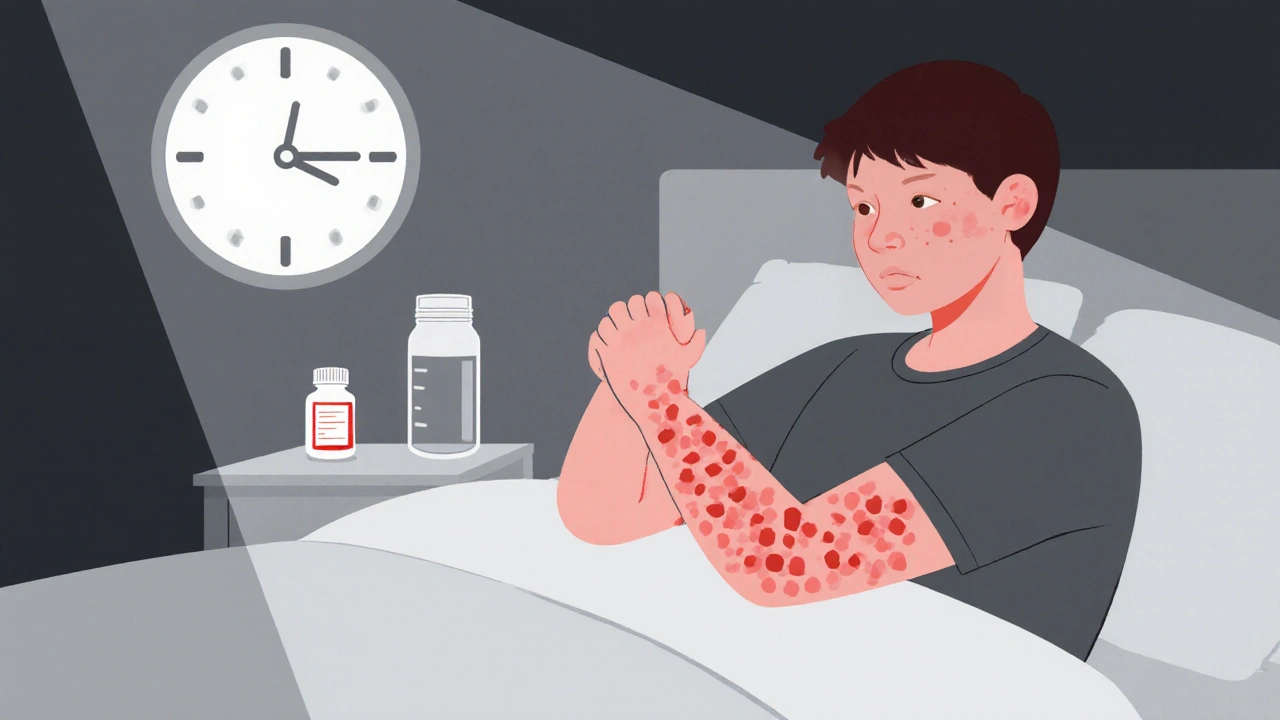
When hives won’t go away - not for weeks, not for months, not for years - you’re not just dealing with a skin rash. You’re living with a disease that steals sleep, ruins confidence, and makes every day a guessing game. Chronic spontaneous urticaria (CSU) affects about 1 in 100 people. For many, the first-line treatment - a daily second-generation antihistamine - helps a little. Maybe even half the time. But for the other 60%, nothing changes. The itching doesn’t stop. The welts keep appearing. And the frustration builds.
Why First-Line Treatments Often Fail
Most doctors start with antihistamines like cetirizine, loratadine, or fexofenadine. They’re safe, cheap, and widely available. But here’s the hard truth: only about 40% of people with CSU get real relief from standard doses. Even if you bump the dose up to two, three, or four times the normal amount - which some doctors do - you still won’t help most patients. The American Academy of Family Physicians acknowledges this. They say the data on higher doses is weak. And honestly? Many patients stop taking them because of drowsiness, dry mouth, or just feeling like it’s not worth it.That’s where second-line treatments come in. These aren’t backup options. They’re the next step for people who’ve already tried everything else. And the goal isn’t just to reduce symptoms. It’s to get them gone - completely.
Omalizumab: The Current Standard, But Not Perfect
For over a decade, omalizumab has been the go-to second-line treatment. It’s an injectable biologic, given once a month under the skin. It works by mopping up IgE antibodies - the ones that trigger mast cells to release histamine and cause hives. In clinical trials, about 30 to 70% of patients saw their symptoms cut in half. That sounds good. Until you realize that 70% of those same patients still didn’t get full control. They still had hives. Still had itching. Still had to plan their lives around flare-ups.And here’s the catch: omalizumab doesn’t work well for everyone. About 30% of CSU cases are driven not by IgE, but by IgG autoantibodies - rogue antibodies that directly activate mast cells. These patients rarely respond to omalizumab. If you’ve been on it for months and nothing changed, you might be in this group. That’s not your fault. It’s just biology.
Doctors in Houston, New York, or Berlin will tell you: omalizumab is still the most common second-line choice. But it’s not the end of the road. It’s just the first real option after antihistamines fail.
Remibrutinib: The Oral Alternative on the Horizon
Imagine taking a pill instead of getting a shot every month. That’s what remibrutinib offers. It’s a Bruton tyrosine kinase inhibitor (BTKi) - a new class of drug that blocks signals inside mast cells and B cells. It doesn’t just calm histamine release. It targets the root cause: the overactive immune cells driving the inflammation.In two large phase 3 trials - REMIX-1 and REMIX-2 - involving more than 900 adults with CSU that didn’t respond to antihistamines, remibrutinib delivered complete symptom control in 28 to 32% of patients after 24 weeks. That’s similar to omalizumab. But here’s the difference: it’s oral. No needles. No clinic visits. Just a daily tablet.
Patients in trials reported better quality of life. Fewer missed workdays. Less anxiety about sudden flare-ups. And because it works on both mast cells and the autoimmune B cells that make the bad antibodies, it might help those who didn’t respond to omalizumab. That’s huge. If approved, remibrutinib could change how CSU is treated - not just because it works, but because it’s easier to take.

Dupilumab: A Biologic That Works Differently
You might know dupilumab from its use in eczema or asthma. It blocks interleukin-4 and interleukin-13 - two key drivers of type 2 inflammation. In CSU, it’s showing promise too. In phase 3 trials, 30 to 31% of patients achieved complete symptom resolution at 24 weeks. That’s slightly better than omalizumab’s average.It’s given as a subcutaneous injection, like omalizumab - but every two weeks. And unlike omalizumab, it doesn’t target IgE. That means it could help patients with IgG-mediated CSU who don’t respond to omalizumab. Early data suggests it might be more effective for those with higher levels of inflammation markers.
The big downside? It’s not yet approved for CSU. Not in the U.S. Not in Europe. But it’s close. The FDA and EMA are reviewing the data. If approved, it could become a first-choice biologic - especially for patients who want something different from omalizumab.
Barzolvolimab and Other Emerging Options
There’s another drug in the pipeline: barzolvolimab. In early trials, it delivered complete response rates of 38 to 51% in just 12 weeks. That’s the highest number seen so far. It’s still in phase 2, so it’s not available yet. But the results are promising.Not all new drugs made it. Fenebrutinib, another BTK inhibitor, was dropped in 2023 after some patients developed liver enzyme spikes. That’s why doctors are cautious. New drugs can be powerful, but safety matters. Every new treatment has to prove it doesn’t trade one problem for another.
Cyclosporine: The Old Workhorse With Big Risks
Before biologics, doctors turned to cyclosporine - an old immunosuppressant used for organ transplants. It’s not FDA-approved for CSU, but many allergists use it off-label. It works well - 54 to 73% of patients see big improvements, especially those with autoimmune CSU. If omalizumab failed you, cyclosporine might help.But it’s not gentle. It can raise blood pressure. It can damage your kidneys. You need regular blood tests. You can’t take it for years. It’s a bridge - not a long-term solution. Still, for someone drowning in hives with no other options, it’s a lifeline.
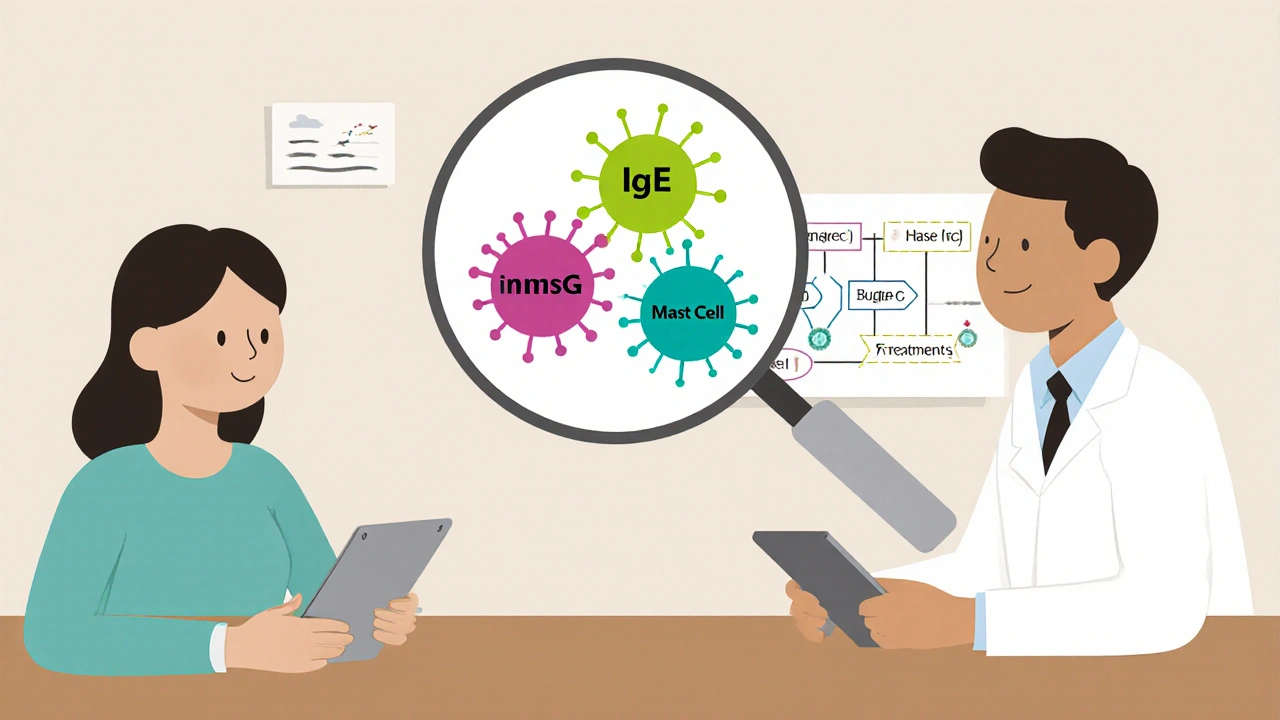
Choosing the Right Path
So how do you pick? There’s no one-size-fits-all. But here’s how doctors are starting to think about it:- If you’re allergic to needles and want something you can swallow - remibrutinib (when available) is the clear winner.
- If you’ve tried omalizumab and it didn’t work - dupilumab or cyclosporine might be next, depending on your lab results.
- If you have high levels of IgG autoantibodies - skip omalizumab. Go straight to dupilumab or cyclosporine.
- If you need fast relief and can’t wait for new drugs - cyclosporine is still the most effective short-term option.
Testing for autoantibodies isn’t routine everywhere. But if your doctor doesn’t offer it, ask. Knowing your CSU subtype isn’t just academic - it changes your treatment plan.
What’s Next?
The CSU treatment landscape is shifting fast. Five years ago, omalizumab was the only option beyond antihistamines. Now, we have at least three strong contenders, with more coming. The future isn’t just about better drugs. It’s about smarter choices. Personalized treatment. Matching the right drug to the right patient based on their immune profile.That’s why the next five years will be critical. If remibrutinib gets approved, it could become the new second-line standard. If dupilumab follows, we might see a two-track system: biologics for one group, oral drugs for another. And for the 1 in 3 patients who don’t respond to any current treatment? Research is already looking at next-gen inhibitors, combination therapies, and even gene-targeted approaches.
You’re not stuck. You’re not alone. And you don’t have to keep living with hives that won’t quit. The tools are getting better. The science is catching up. The question now isn’t whether there’s hope - it’s which path is right for you.
What if my antihistamines don’t work at all?
If standard-dose antihistamines don’t help, don’t wait months to try something else. Most guidelines recommend moving to second-line treatment after 2-4 weeks if symptoms persist. Increasing the dose helps only about 40% of people. If you’re still breaking out after that, it’s time to talk to your doctor about omalizumab, remibrutinib (when available), or other options. Waiting longer doesn’t make the hives go away - it just makes life harder.
Is omalizumab worth the cost and injections?
For many, yes - but only if it works. Omalizumab costs thousands per month, and you need monthly shots. If you get partial relief - say, your hives drop from daily to twice a week - it might be worth it. But if you’ve been on it for 3-6 months and still have constant symptoms, it’s not working for you. That’s not failure. It just means your CSU is driven by something omalizumab can’t touch, like IgG autoantibodies. Talk to your doctor about switching.
Can I take remibrutinib and omalizumab together?
No - and you shouldn’t need to. Both drugs target the same immune pathway in different ways. Taking them together increases risk without proven benefit. The goal is to find one that works. If omalizumab failed, remibrutinib is the next logical step. If remibrutinib isn’t available yet, dupilumab or cyclosporine are better alternatives than combining treatments.
How do I know if I have autoimmune CSU?
There’s a simple blood test called the autologous serum skin test (ASST) or an IgG autoantibody panel. These aren’t done in every clinic, but they’re available at major allergy centers. If you’ve had CSU for over a year and didn’t respond to omalizumab, you’re more likely to have autoimmune CSU. Ask your doctor to order the test - it changes your treatment options.
Are these new drugs safe for long-term use?
Omalizumab has 10+ years of safety data - it’s well tolerated. Dupilumab, used for years in eczema, shows no major long-term risks. Remibrutinib’s trials lasted 24 weeks, and side effects were mild - mostly headaches and upper respiratory infections. Cyclosporine is the only one with clear long-term risks (kidney, blood pressure). For newer drugs like remibrutinib and barzolvolimab, we’ll know more in 2-5 years as more patients use them. But so far, the safety profile looks promising.
What to Do Next
If you’re stuck in the cycle of hives and ineffective meds, here’s your action plan:- Track your symptoms for 2 weeks - note frequency, severity, triggers (if any), and how antihistamines affect you.
- Ask your doctor if you’ve tried the highest safe dose of antihistamines. If not, give it 2-4 weeks.
- If still no relief, ask about testing for IgG autoantibodies.
- Based on results, discuss: omalizumab (if negative), dupilumab (if available), or cyclosporine (if urgent).
- Stay updated - remibrutinib could be approved by late 2026. Ask your doctor if you’re eligible for clinical trials.
You’re not broken. Your immune system is just misfiring. And now, we have more ways to fix it than ever before.

Swati Jain
November 22, 2025 AT 13:37Let’s be real - if your doctor still thinks antihistamines are the endgame for CSU, they’re operating on 2012 guidelines. Omalizumab isn’t a ‘second-line’ - it’s the bare minimum. And now? We’ve got remibrutinib, an oral BTKi that actually targets mast cell signaling, not just IgE. This isn’t incremental. It’s a paradigm shift. Stop settling for ‘less itching’ and demand complete remission. Your quality of life isn’t negotiable.
Florian Moser
November 23, 2025 AT 10:20This is one of the most clear, clinically accurate summaries of CSU management I’ve seen outside of a journal. The distinction between IgE-driven and IgG-mediated disease is critical, and too often overlooked. For patients who’ve endured years of ineffective treatment, knowing that dupilumab or cyclosporine may be more appropriate than continuing omalizumab is empowering. Thank you for highlighting actionable next steps.
jim cerqua
November 23, 2025 AT 18:20OH MY GOD. I’ve been on omalizumab for 18 months. ZERO improvement. I’ve cried in dermatology waiting rooms. I’ve canceled weddings. I’ve worn long sleeves in 95-degree heat just to hide the welts. And now you’re telling me it’s because my immune system is just… rogue? Like, not broken - just叛逆? I’m not a failure. My body’s just been playing chess while my doctor was playing checkers. I’m crying. Again. But this time, it’s because I finally feel seen.
Donald Frantz
November 24, 2025 AT 03:53Why is dupilumab not approved yet for CSU? The phase 3 data is robust. The safety profile is better than cyclosporine. The mechanism is distinct from omalizumab. The FDA is dragging its feet. Is it because biologics are too profitable to rush? Or because the pharmaceutical lobby is protecting omalizumab’s market share? Someone’s利益 is being prioritized over patient outcomes. This isn’t science - it’s bureaucracy.
Sammy Williams
November 25, 2025 AT 08:01Man, I’ve been in this hell for 5 years. I tried everything. Antihistamines? Meh. Omalizumab? Felt like throwing money down a well. Then my allergist said, ‘Try cyclosporine - it’s rough, but it works.’ Two weeks in, my skin looked normal for the first time since 2019. Yeah, I have to get bloodwork every month. Yeah, I’m nervous about my kidneys. But I slept through the night last week. That’s worth it. Don’t let fear stop you from asking for the hard options.
Julia Strothers
November 25, 2025 AT 23:13Remibrutinib? Dupilumab? All these ‘new’ drugs? I’ve seen this before. They come out, the media goes nuts, then people start dying from ‘off-label’ use. Remember Vioxx? Thalidomide? This is Big Pharma’s new scam. They’re selling hope to desperate people so they can charge $10,000/month. And guess who pays? YOU. The FDA is a puppet. Your doctor’s just another sales rep. Wake up. This isn’t medicine - it’s a Ponzi scheme dressed in lab coats.
Erika Sta. Maria
November 27, 2025 AT 14:15But like… what if the whole autoimmune model is wrong? I mean, hives are just… surface noise. What if the real issue is gut dysbiosis? Or glyphosate? Or 5G triggering mast cell degranulation? I read this one guy on Medium who said CSU is just your body screaming because you ate too much gluten in 2017. And he had a PhD in ‘spiritual immunology’? I think we’re missing the forest for the IgE trees. Also, omalizumab is just a placebo with a fancy name.
Nikhil Purohit
November 28, 2025 AT 09:47Swati nailed it - this isn’t about ‘second-line’ anymore. It’s about precision medicine. If you’re IgG-positive and on omalizumab, you’re wasting time. The ASST test is cheap, non-invasive, and available at most academic centers. If your doc says ‘we don’t do that here,’ ask for a referral. Also, remibrutinib’s 32% complete response rate? That’s not ‘good enough’ - it’s revolutionary. For the first time, we have an oral option that doesn’t just suppress - it reprograms. And yes, cyclosporine still has a place. It’s not pretty, but it’s effective. Use it as a bridge, not a life sentence.
Debanjan Banerjee
November 30, 2025 AT 06:49Just came back from my allergist. I’ve been on omalizumab for 14 months. No change. I asked about dupilumab and IgG testing. She looked at me like I asked for a unicorn. Said ‘we don’t do that here.’ So I went to a university clinic. Got the test. IgG-positive. Two weeks later, I started dupilumab. Week 3: zero hives. Zero itching. I slept. I wore shorts. I laughed. This isn’t luck. It’s science. If your doctor won’t listen - find a new one. Your life is worth more than their inertia.
Steve Harris
December 2, 2025 AT 06:33I appreciate the depth here. The balance between optimism and realism is rare. I’ve been on cyclosporine for 6 months - it’s kept me functional, but I’m scared of the long-term risks. I’m waiting for remibrutinib’s approval. In the meantime, I’m tracking my symptoms religiously and pushing for the IgG panel. I don’t want to be a statistic. I want to be a success story. Thank you for giving me the language to advocate for myself.
Michael Marrale
December 2, 2025 AT 20:47Wait - so you’re telling me there’s a pill that could fix this? And I’ve been getting shots for years? Why didn’t anyone tell me this before? I feel like I’ve been lied to. Like my whole life has been a marketing campaign for Big Pharma. I’m gonna go cry in my car. But also… I’m kinda excited? Like… maybe I’m not broken? Maybe it’s just the wrong tool? This changes everything.
David vaughan
December 3, 2025 AT 05:57Just wanted to say: thank you. I’ve been reading this post three times. I’ve been crying. I’ve been printing it out. I’ve been showing it to my husband. I thought I was alone. I thought I was crazy. But now I know - there’s a path. And I’m not giving up. I’m getting the test. I’m asking for dupilumab. I’m not settling for ‘less itchy.’ I deserve to be free.
David Cusack
December 4, 2025 AT 08:17