Medication Side Effect Risk Calculator
Medication Safety Assessment
Women are nearly twice as likely as men to have a bad reaction to the same dose of a medication. It’s not just bad luck. It’s biology, history, and a system built on data that mostly ignored them.
Biological Differences That Change How Drugs Work
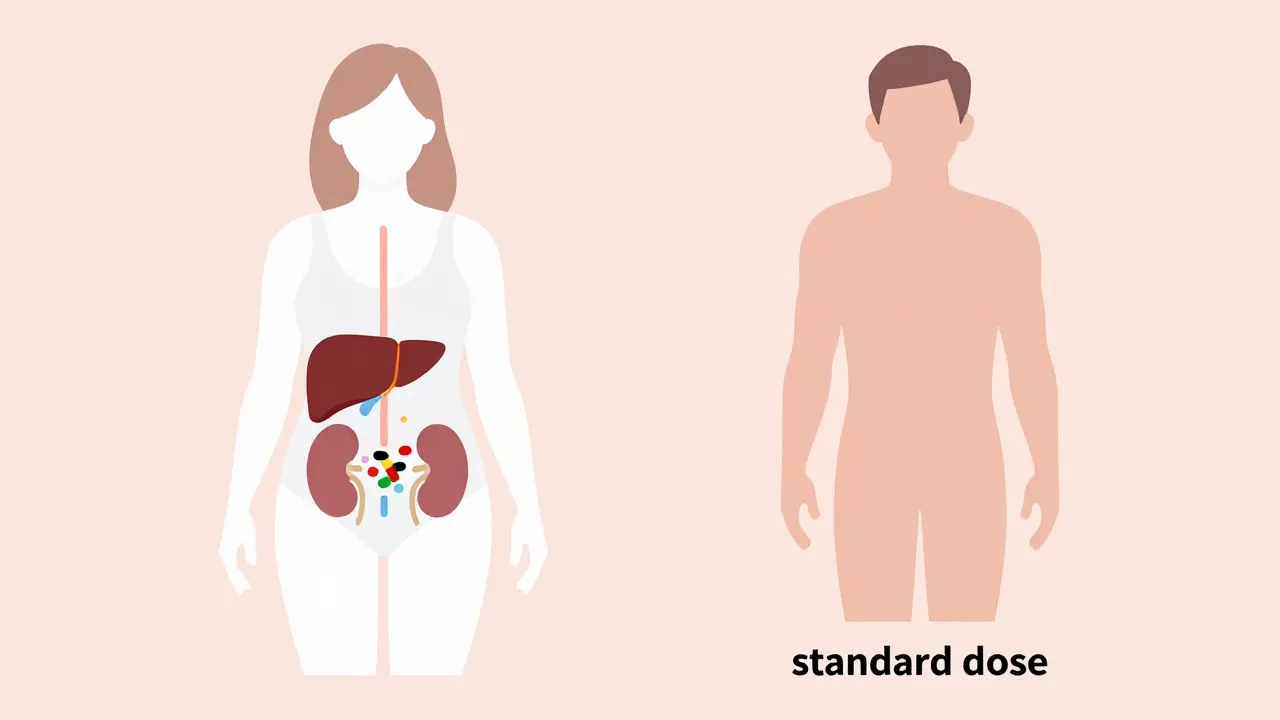
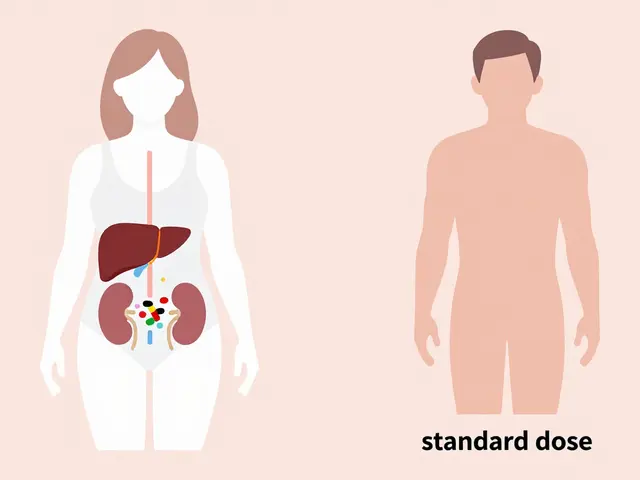
Your body processes medicine differently based on sex-and those differences are measurable. Women have, on average, 40% less of the liver enzyme CYP3A4 than men. This enzyme breaks down about half of all prescription drugs, including common ones like statins, benzodiazepines, and antidepressants. Slower breakdown means the drug sticks around longer, building up in the system and increasing the chance of side effects. Body composition plays a big role too. Women typically have 10-12% more body fat than men. That matters because fat-soluble drugs like diazepam (Valium) or antidepressants get stored in fat tissue. They don’t clear out as quickly, so women end up with higher concentrations in their blood over time. At the same time, women’s kidneys clear drugs like lithium and some antibiotics 20-25% slower than men’s. Even small changes in how fast a drug leaves the body can turn a safe dose into a dangerous one. Hormones add another layer. Birth control pills can slash the effectiveness of the seizure drug lamotrigine by up to 60%, forcing women to adjust their doses. During different phases of the menstrual cycle, metabolism can shift by up to 30%. That means the same pill taken on day 5 versus day 20 could have wildly different effects. Yet most prescriptions don’t account for this.The Zolpidem Case: When the FDA Finally Acted
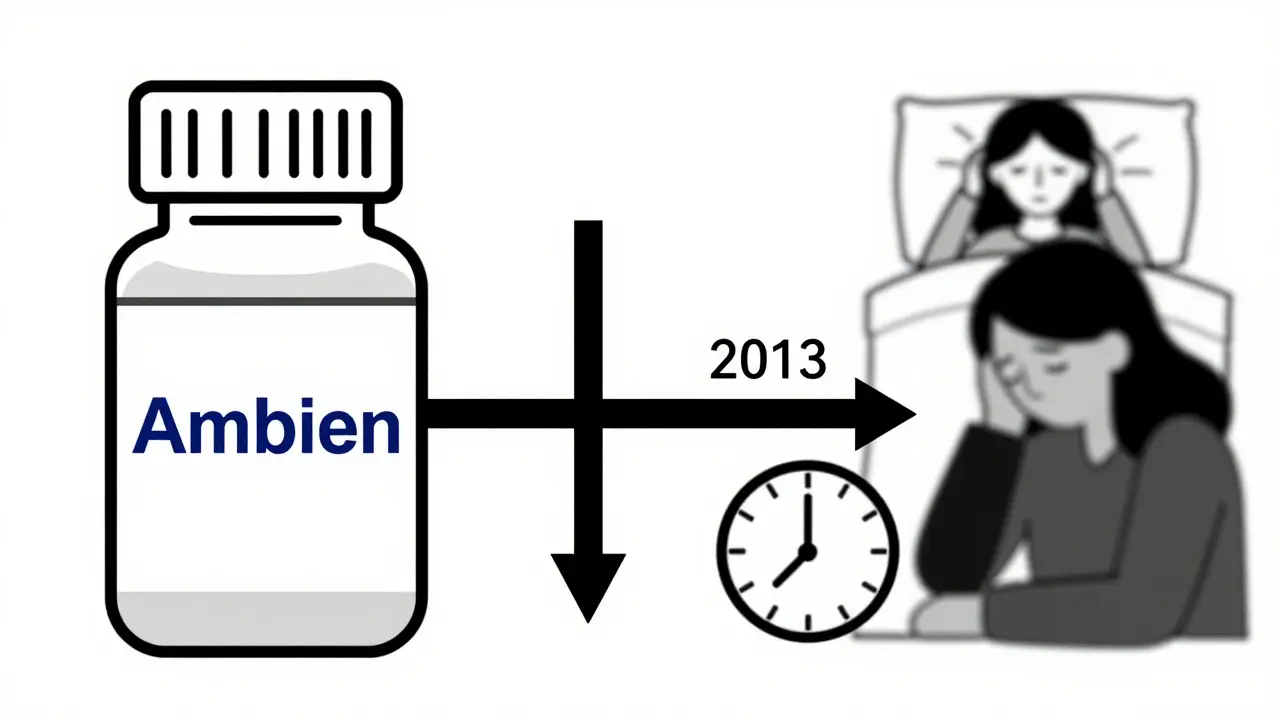
One of the clearest examples of this gap is zolpidem, sold as Ambien. In the 1990s, studies showed women metabolized it 50% slower than men. They woke up groggy, disoriented, even impaired enough to drive dangerously. Yet the same dose was given to everyone. It wasn’t until 2013-over 20 years after the first warning-that the FDA required a 50% lower dose for women. The result? Adverse event reports from women dropped by 38% within five years. That’s not a coincidence. It’s proof that adjusting for biological reality saves lives. But Ambien is the exception, not the rule. Out of 86 FDA-approved medications with known sex-based differences in metabolism, only 15 have sex-specific dosing instructions on their labels.What Side Effects Are Women More Likely to Get?
Women don’t just get side effects more often-they get different ones.- With SSRIs like sertraline or fluoxetine, women report 1.5 to 2 times more nausea and dizziness.
- Antipsychotics like haloperidol cause QT prolongation-a heart rhythm issue-2.3 times more often in women.
- Antibiotics like sulfamethoxazole trigger severe skin reactions in women 47% more frequently.
- On opioids, women are 2.1 times more likely to report severe side effects and are far more likely to stop taking them because of them.
Why Is This Happening? The History Behind the Gap
The root of this problem goes back to the 1970s. Fearing harm to unborn babies, the FDA banned women of childbearing age from early drug trials. That policy lasted decades. Even after it was officially reversed in 1993, progress was slow. By 2022, only 12% of pharmacokinetic studies-the ones that track how drugs move through the body-analyzed results by sex. So for years, drug doses were set based on how men responded. The average male body became the default. Women were treated as if they were just smaller men. But they’re not. Their bodies work differently. And the consequences are real: an estimated $30 billion a year in U.S. healthcare costs from preventable adverse reactions, mostly in women.Are Biological Differences the Whole Story?
Not everyone agrees. Some researchers argue that women aren’t biologically more sensitive-they’re just more likely to report symptoms and seek care. Harvard’s Sarah Richardson analyzed 33 million FDA reports and found that once you account for the fact that women take 56% more prescriptions than men, the sex-based gap in adverse events shrinks to under 5%. That’s a powerful point. Women are more likely to notice side effects. They’re more likely to call their doctor. They’re more likely to fill out surveys. Men often tough it out. That doesn’t mean biology isn’t real-it just means we can’t ignore reporting bias. NIH’s Dr. Janine Austin Clayton puts it plainly: both factors matter. Biological differences exist. But so does the way we ask questions, collect data, and interpret results.Doctors Don’t Know What They Don’t Know
Even when the science is clear, it doesn’t reach the clinic. A 2022 AMA survey found only 28% of physicians routinely consider sex differences when prescribing common medications. Two-thirds didn’t even know about the FDA’s 2013 zolpidem dose change. Drug labels are the main source of prescribing guidance-and most don’t mention sex. Only 4% of drug labels include sex-specific dosing advice. That leaves doctors guessing. If a woman has a bad reaction to a standard dose, many assume it’s an allergy, not a metabolic issue. So they stop the drug instead of lowering the dose. And when patients do speak up, they’re often dismissed. On Reddit, nurses and patients describe how women’s complaints about dizziness or nausea are labeled as “anxiety” or “overreacting.” Meanwhile, men’s side effects are taken at face value.
What’s Changing-and What’s Not
There’s movement, but it’s uneven. The European Medicines Agency now requires sex-stratified data in all Phase III trials. The FDA launched its “Sex and Gender Roadmap” in 2023, aiming to make sex a standard part of drug evaluation by 2026. The NIH is funding $12.5 million in new research on sex differences at Harvard. Projects like the University of California’s JUST Dose study are using AI to build personalized dosing models based on sex, weight, age, and hormones. Early results show a 40% drop in side effects when sex-specific dosing is used. But progress is slow. Only 32% of cardiovascular drug trials analyze results by sex. Less than 0.5% of pharmaceutical R&D is focused on sex-specific formulations. And while the global women’s health market is growing at 8.7% a year, it’s still only 3.2% of the total pharmaceutical industry.What You Can Do
If you’re a woman taking medication:- Ask: “Was this dose tested on women?”
- Track side effects: note when they happen, how bad they are, and if they change with your cycle.
- If you feel worse than expected, don’t assume it’s you. Ask about dose reduction.
- Use tools like the FDA’s Drug Trials Snapshots to see if sex-specific data was reported.
- Start asking about sex differences in every prescription.
- Know which drugs have known sex-based metabolism differences (zolpidem, digoxin, lamotrigine, SSRIs, statins).
- Consider starting low and going slow-especially with women.
The Bottom Line
This isn’t about men vs. women. It’s about accuracy. Medicine works best when it’s tailored. For decades, we treated women like men with extra body fat. Now we know that’s not just wrong-it’s dangerous. The science is here. The data is clear. The fixes are simple: start with lower doses. Analyze results by sex. Update labels. Train doctors. Make sex a standard variable-not an afterthought. The cost of doing nothing? Billions in wasted healthcare dollars. Thousands of preventable hospital visits. And women who are told their symptoms are “in their head” when they’re really in their biology. It’s time to stop treating half the population as outliers.Why do women have more side effects from medication than men?
Women have lower levels of key liver enzymes that break down drugs, higher body fat that stores fat-soluble medications, slower kidney clearance, and hormonal fluctuations that affect drug metabolism. These biological differences mean drugs stay in women’s systems longer and at higher concentrations, increasing side effect risk. Historical exclusion from clinical trials means most dosing was based on male physiology, leaving women underdosed or overdosed.
What medications are known to affect women differently?
Zolpidem (Ambien), digoxin, lamotrigine, SSRIs like sertraline and fluoxetine, statins, benzodiazepines, lithium, and some antibiotics like sulfamethoxazole all show significant sex-based differences. For example, women metabolize zolpidem 50% slower, leading to FDA-mandated dose reductions. Women also experience 2.3 times more QT prolongation from antipsychotics and 47% higher risk of severe skin reactions from antibiotics.
Did the FDA fix the problem with Ambien?
Yes. In 2013, after decades of evidence showing women metabolized zolpidem slower, the FDA cut the recommended dose for women by 50%. Post-market data showed a 38% drop in adverse events among women within five years. This remains one of the few successful examples of sex-specific dosing being implemented.
Are women just reporting side effects more often?
Partly. Women are more likely to seek care, report symptoms, and participate in surveys. One study found that once you account for the fact that women take 56% more prescriptions, the gap in adverse event rates drops significantly. But that doesn’t erase biological differences-both factors are real. The issue isn’t whether women report more-it’s whether we’ve been ignoring the biology behind their reports.
Should women always take lower doses of medication?
Not always-but they should start lower, especially with drugs known to have sex-based metabolism differences. For zolpidem, digoxin, and SSRIs, starting at a reduced dose and adjusting based on response is safer. The goal isn’t to assume all women need less, but to avoid the default male-based dose that may be too high. Always discuss options with your provider.
Is this issue getting better?
Slowly. The FDA and EMA now require sex-stratified data in trials. New research initiatives are using AI to build sex-specific dosing models. But only 4% of drug labels include sex-based dosing advice, and most doctors aren’t trained on these differences. Real change requires mandatory reporting, updated labels, and provider education-none of which are happening fast enough.

Michael Camilleri
January 24, 2026 AT 17:39Also, if you're a woman on SSRIs and you're dizzy as hell, maybe it's not anxiety. Maybe it's your liver working at half speed. Stop blaming yourself.
Darren Links
January 25, 2026 AT 15:44Helen Leite
January 27, 2026 AT 15:12Elizabeth Cannon
January 28, 2026 AT 07:01Start low, go slow. It's not rocket science. But it's like asking a rock to do yoga. Nobody wants to change the script.
Phil Maxwell
January 29, 2026 AT 20:20Tommy Sandri
January 31, 2026 AT 07:18Sushrita Chakraborty
February 1, 2026 AT 20:49Amelia Williams
February 3, 2026 AT 07:09venkatesh karumanchi
February 4, 2026 AT 23:39Jenna Allison
February 5, 2026 AT 23:03Also-track your cycle. If you feel worse on day 20? That's not coincidence. That's estrogen.
Sharon Biggins
February 6, 2026 AT 03:55John McGuirk
February 7, 2026 AT 22:08lorraine england
February 8, 2026 AT 14:25