Fibrate Choice Advisor
Compare Gemfibrozil vs Fenofibrate
Answer a few questions to determine which fibrate may be more appropriate for your situation.
Recommendation
Gemfibrozil
Fenofibrate
When it comes to lowering stubborn triglycerides, two drugs often surface: Gemfibrozil is a fibric acid derivative that activates PPAR‑α to boost lipid breakdown and Fenofibrate is another fibric acid agent, also a PPAR‑α agonist, but formulated in several salt forms. Both aim to trim high triglycerides, yet their dosing, side‑effect profile, and interaction risk differ enough that doctors-and patients-need a clear side‑by‑side rundown.
How the two medicines work
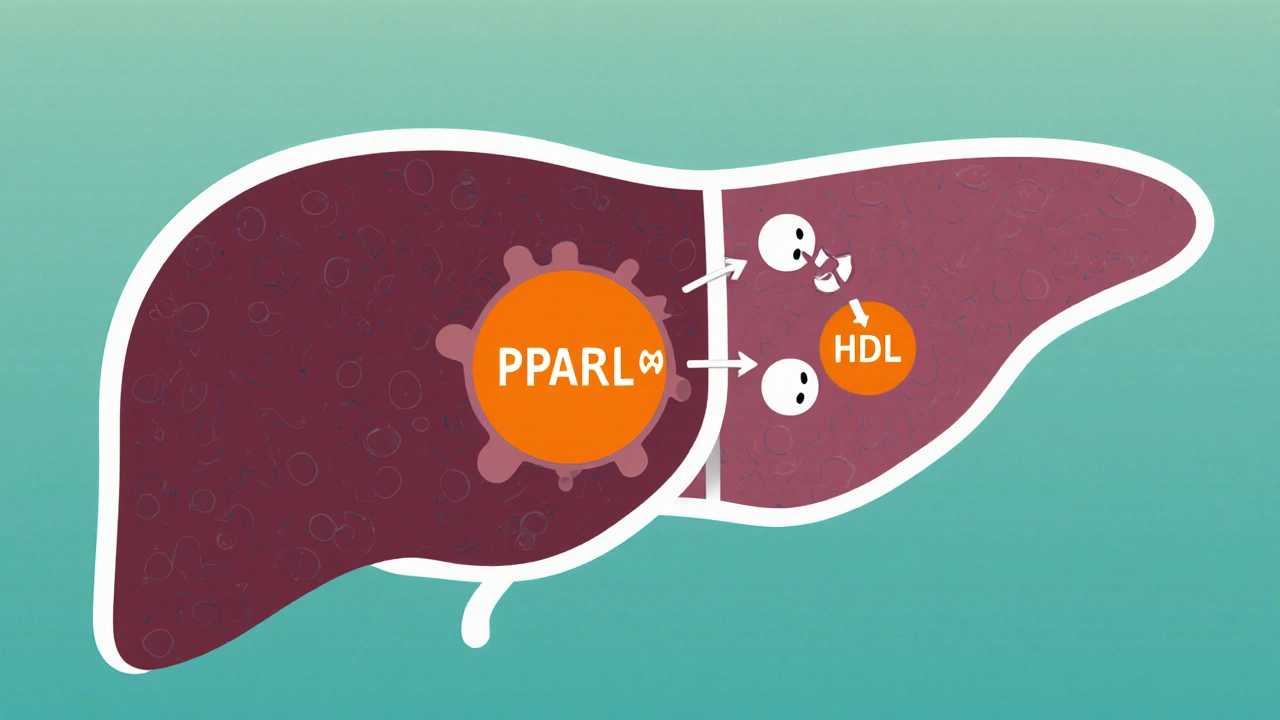
Both drugs belong to the fibrate class, which means they bind to the peroxisome proliferator‑activated receptor‑alpha (PPAR‑α). Activation of this nuclear receptor turns on genes that increase the breakdown of very‑low‑density lipoprotein (VLDL) and boost the production of high‑density lipoprotein (HDL). In everyday language, they help the liver mop up excess triglycerides and lift the “good” cholesterol.
Where they diverge is mostly in chemistry. Gemfibrozil is a single molecule, while fenofibrate is sold as several salts (micronized fenofibrate, fenofibric acid, etc.). Those salts affect how quickly the drug dissolves, its bioavailability, and consequently the dose needed for the same effect.
Approved uses and when doctors choose one over the other
- Gemfibrozil: FDA‑approved for severe hypertriglyceridemia (≥500 mg/dL) and for mixed dyslipidemia when statins alone are insufficient.
- Fenofibrate: Approved for similar indications but also for primary hypercholesterolemia when combined with a statin, and for patients with diabetic dyslipidemia who have high triglycerides and low HDL.
Because fenofibrate’s salt forms improve absorption, many clinicians prefer it when patients need a lower pill burden or have trouble reaching therapeutic levels with gemfibrozil.
Typical dosing schedules
- Gemfibrozil - usually 600 mg twice daily with meals. The split dose helps reduce gastrointestinal upset.
- Fenofibrate - varies by formulation:
- Micronized tablets: 145 mg once daily with food.
- Fenofibric acid (Trilipix): 135 mg once daily.
Both drugs should be taken with meals to improve absorption and to lessen stomach irritation.
Side‑effect profiles - what to watch for
While the class‑wide side effects overlap, subtle differences matter in practice.
| Common GI upset (nausea, dyspepsia) | Both |
| Myalgia or muscle pain | More frequent with gemfibrozil, especially when combined with statins |
| Elevated liver enzymes (ALT, AST) | Both, but fenofibrate tends to cause milder elevations |
| Kidney stone formation | Rare, reported more often with gemfibrozil |
Both drugs can increase the risk of gallstones because they raise bile cholesterol saturation.
Drug‑interaction warnings
Knowing which interactions are real versus theoretical can save a patient from unnecessary dose cuts.
- Statins: Gemfibrozil strongly inhibits statin metabolism (especially simvastatin and lovastatin), raising the chance of rhabdomyolysis. Fenofibrate has a milder effect, so many doctors feel safer pairing it with low‑dose rosuvastatin or pravastatin. \n
- Anticoagulants: Both fibrates can enhance the effect of warfarin; INR must be monitored more closely after initiation.
- Oral hypoglycemics: Fenofibrate may modestly increase blood glucose; patients on sulfonylureas may need dose adjustments.
- Cyclosporine: Increases fenofibrate levels; avoid unless benefits outweigh risks.
Always review a patient’s full medication list before starting either drug.
Renal and hepatic considerations
Both agents are processed by the liver and excreted via kidneys, but the balance differs.
- Kidney disease: Gemfibrozil requires dose reduction when creatinine clearance < 30 mL/min. Fenofibrate’s micronized form can be used with caution down to 30 mL/min, but the acid form needs a 50 % dose cut.
- Liver disease: Neither drug is recommended for active hepatitis or severe cirrhosis. Mild ALT elevations are acceptable if they stay under three times the upper limit.
Cost and insurance coverage (2024‑2025 snapshot)
Insurance formularies still list both drugs, but price gaps exist.
- Gemfibrozil (generic) - average retail $0.10‑$0.15 per 600 mg tablet.
- Fenofibrate (generic salts) - average retail $0.20‑$0.35 per tablet, with brand‑name Tricor costing up to $1.20 per dose.
For patients paying out‑of‑pocket, gemfibrozil is usually the cheaper choice, but the higher pill burden and interaction risk may offset the savings.
How to decide which drug fits you best
Below is a quick decision matrix you can print or save.
| Attribute | Gemfibrozil | Fenofibrate |
|---|---|---|
| Drug class | Fibrate (single molecule) | Fibrate (salt forms) |
| Mechanism | PPAR‑α activation | PPAR‑α activation |
| FDA‑approved uses | Severe hypertriglyceridemia, mixed dyslipidemia | Hypertriglyceridemia, diabetic dyslipidemia, statin‑add‑on therapy |
| Typical dose | 600 mg BID | 145 mg daily (micronized) or 135 mg daily (acid) |
| Half‑life | ~1.5 h (requires BID) | ~20 h (once daily) |
| Main side effects | GI upset, muscle pain (with statins), kidney stones | GI upset, mild liver enzyme rise, possible gallstones |
| Contraindications | Severe liver disease, active gallbladder disease | Severe liver disease, severe renal impairment (<30 mL/min) |
| Notable interactions | Statins (esp. simvastatin), warfarin, anticoagulants | Statins (lower risk), warfarin, cyclosporine, sulfonylureas |
| Average US price (2024) | $0.12 per tablet | $0.28 per tablet (generic) |
In a nutshell, pick gemfibrozil if cost is the overriding concern and the patient isn’t on a high‑dose statin. Choose fenofibrate for better adherence, milder drug‑interaction profile, and when the patient already takes a statin.
Frequently asked questions
Can I switch from gemfibrozil to fenofibrate?
Yes, but you should taper off gemfibrozil over a few days to avoid a sudden drop in triglyceride control. Your doctor will start fenofibrate at the recommended dose and monitor liver enzymes and kidney function during the transition.
Is it safe to take either drug during pregnancy?
Both gemfibrozil and fenofibrate are classified as Category C - animal studies have shown some risk, and there are no well‑controlled human studies. They should be used only if the potential benefit outweighs the risk, and always under close obstetric supervision.
Why do some labs report a rise in creatine kinase (CK) with gemfibrozil?
Gemfibrozil interferes with the metabolism of certain statins, leading to higher plasma levels of the statin and, consequently, muscle toxicity. Elevated CK is a red flag for rhabdomyolysis, so clinicians often avoid the combo or use a statin with a lower interaction risk such as rosuvastatin.
Do either of these drugs affect blood sugar?
Fenofibrate can modestly increase fasting glucose, so diabetic patients need periodic monitoring. Gemfibrozil has a neutral effect on glucose for most people.
Bottom line
Both gemfibrozil and fenofibrate belong to the same drug family, but their chemical forms, dosing convenience, interaction risk, and cost differ enough to matter in real‑world treatment plans. Understanding the nuances helps patients and clinicians pick the right tool for the job, keeping triglycerides low while avoiding unnecessary side effects.



Michael Vandiver
October 22, 2025 AT 18:31Great rundown thanks for the clear comparison! 😊
Vin Alls
October 26, 2025 AT 18:44What a vivid tapestry of pharmacology! The way gemfibrozil and fenofibrate each tug at the PPAR‑α lever is like watching two conductors leading the same orchestra, yet their scores differ. Gemfibrozil’s split‑dose rhythm can be a hassle, but its punchy statin interaction makes it a heavyweight in certain cases. Fenofibrate, especially in its micronized guise, glides in once daily, offering a smoother ride for patients who dread pill piles. Bottom line: match the drug’s chemistry to the patient’s lifestyle and comorbid meds, and you’ll strike a harmonious lipid balance.
Tiffany Davis
October 30, 2025 AT 06:04I appreciate the clear layout of the dosing schedules. The side‑effect nuances, especially the muscle pain risk with gemfibrozil, are worth noting. Overall, the choice really hinges on the patient’s other medications and kidney function.
Sajeev Menon
November 2, 2025 AT 03:31Hey folks, just wanted to add a quick mentor note – when you’re looking at renal dosing, don’t forget that gemfibrozil needs a cut when CrCl drops below 30 mL/min, while fenofibrate’s micronized form can still work down to about the same level. Also, keep an eye on liver enzymes; mild upticks are normal but anything over three times the ULN should raise a flag. If you’re mixing with statins, gemfibrozil is the one that can definately boost the risk of rhabdomyolysis – so monitor CK regularly.
Emma Parker
November 4, 2025 AT 11:04Yo, great post! I think most peeps forget how cheap gemfibrozil is – that price tag can really matter.
Joe Waldron
November 6, 2025 AT 04:44Excellent points, Sajeev; indeed, the renal thresholds are critical, and I would add that the pharmacokinetic half‑life of gemfibrozil (~1.5 h) necessitates the BID regimen, whereas fenofibrate’s ~20 h half‑life supports once‑daily dosing, which can improve adherence. Moreover, clinical guidelines recommend monitoring CK levels at baseline and periodically, especially when statins are co‑administered; this is vital to mitigate myopathy risk. Finally, consider patient education about potential gallstone formation – a rarely discussed but relevant adverse effect.
Wade Grindle
November 7, 2025 AT 14:04I concur with Emma’s observation regarding cost. From a health‑policy perspective, the lower price of gemfibrozil can improve formulary placement, yet clinicians must balance that against its interaction profile.
Benedict Posadas
November 8, 2025 AT 17:51Totally agree, Michael! The comparison is super helpful – especially the side‑effect table 😊. If you’re new to these meds, just remember to take them with meals to cut down the stomach upset – it really makes a diff ;)
Jai Reed
November 9, 2025 AT 16:04When deciding between gemfibrozil and fenofibrate the clinician must first evaluate the patient’s triglyceride burden and any concurrent statin therapy. Gemfibrozil’s twice‑daily schedule can be inconvenient, yet its stronger statin interaction profile demands careful monitoring for myopathy. Fenofibrate’s once‑daily dosing is a major adherence advantage, especially for patients juggling multiple pills. However, the various salt forms of fenofibrate introduce variability in bioavailability that can affect lipid targets. In patients with moderate renal impairment, dose adjustment of gemfibrozil is mandatory, while fenofibrate may be tolerated down to a lower creatinine clearance with the micronized formulation. Liver function must be checked before initiating either drug, as both can cause reversible enzyme elevations. The risk of gallstone formation, though rare, is present with both agents due to increased cholesterol saturation in bile. When combined with anticoagulants, both fibrates potentiate warfarin effects, so INR checks become more frequent. For diabetic patients, fenofibrate offers a modest benefit on fasting glucose, a factor that can tilt the balance toward its use. Cost considerations still favor generic gemfibrozil, but insurance formularies often place fenofibrate on preferred tiers. Real‑world studies have shown that patients on fenofibrate report fewer gastrointestinal complaints than those on gemfibrozil. Nevertheless, the muscle pain risk with gemfibrozil rises sharply when paired with simvastatin or lovastatin. Switching to a low‑dose rosuvastatin can mitigate this risk while preserving lipid control. Ultimately the decision hinges on individual risk factors, pill burden tolerance, and the prescribing clinician’s familiarity with each drug’s interaction profile. I recommend a shared decision‑making conversation that weighs these elements head‑on before committing to therapy.
Sameer Khan
November 10, 2025 AT 08:44The deliberative framework you outlined aligns with current lipid‑management algorithms, particularly the integration of fibrates in the context of mixed dyslipidemia. It is imperative to conduct a risk‑benefit stratification, incorporating pharmacodynamic interaction indices and renal clearance thresholds. Moreover, the pharmacoeconomic implications you mentioned necessitate a formulary‑driven decision matrix, especially when considering generic versus brand‑name entities. The emphasis on shared decision‑making resonates with patient‑centred care models, fostering adherence and therapeutic outcome optimization.
WILLIS jotrin
November 10, 2025 AT 22:37Interesting perspectives all around – it’s clear that both drugs have their niches, and the clinician’s judgment ultimately ties everything together.