When a pharmacist sees a brand-name prescription, they don’t just fill it-they evaluate. Is there a generic version? Is it safe? Is it appropriate for this patient? And if so, how do they tell the prescriber?
This isn’t just about saving money. It’s about making sure patients get the right drug, at the right time, without unnecessary barriers. The reality is that 97% of prescriptions filled in the U.S. are for generics, saving the system over $409 billion a year. But behind every generic filled is a conversation-sometimes quick, sometimes complex-that can make or break adherence, safety, and trust.
When Substitution Is Allowed-and When It’s Not
Most states let pharmacists swap a brand drug for a generic unless the prescriber writes "dispense as written" (DAW) or "do not substitute" (DNS). That’s the law in 49 states. But in 17 states, pharmacists also need to get the patient’s consent before switching. And five states-Connecticut, Massachusetts, New York, Texas, and Virginia-have strict formularies that only allow substitution for specific generic brands listed in state guidelines.
So what triggers a pharmacist to reach out? It’s not just about cost. The real red flags come from clinical risk.
Narrow therapeutic index (NTI) drugs are the biggest concern. These are medications where even a tiny change in dose can cause serious harm. Think warfarin, levothyroxine, phenytoin. The FDA doesn’t treat these the same as other generics. Even though they meet bioequivalence standards, some prescribers worry about switching. Pharmacists know this-and they don’t assume. They check the Orange Book, review the patient’s history, and often call the prescriber to confirm.
Another hidden issue? Inactive ingredients. Generics must have the same active ingredient as the brand-but they can differ in fillers, dyes, or preservatives. For about 8.7% of patients with documented allergies or sensitivities, that difference matters. One patient developed a rash after switching from a brand-name pill to a generic that used a different dye. The pharmacist caught it because they checked the patient’s allergy record before dispensing. They called the prescriber. The next refill went back to the original brand.
The FDA Orange Book: The Pharmacist’s Bible
Every pharmacist uses the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations-better known as the Orange Book. It’s not a suggestion. It’s the official guide for whether a generic is truly interchangeable.
The Orange Book rates drugs with an "A" or "B." An "A" rating means the generic is therapeutically equivalent to the brand. About 92.7% of generics get this rating. A "B" rating means there’s no proven equivalence-those shouldn’t be substituted without a clear clinical reason.
Pharmacists don’t guess. They look up the active ingredient. They check the rating. They compare the manufacturer. If the generic is rated "A," they know they can recommend it. But if it’s "B," or if there’s no rating at all, they pause. They don’t substitute. They call the prescriber.
The Orange Book also lists over 1,456 unique active moieties and 12,876 approved generic products. That’s a lot of data. And it’s updated every month. Pharmacists who stay current on these updates are the ones who reduce errors and build trust with prescribers.
Why Prescribers Hesitate-And How Pharmacists Bridge the Gap
Despite the data, many prescribers still have doubts. A 2023 survey found that 37.6% of doctors worry about generic effectiveness. For inhalers? That number jumps to 42.3%. For topical creams? 38.9%.
Why? Because they’ve seen patients report "it just doesn’t work the same." Sometimes, it’s a real issue. Sometimes, it’s a placebo effect. Sometimes, it’s a formulation difference they don’t understand.
Pharmacists who succeed don’t say, "It’s the same drug." They say, "Here’s what the data shows."
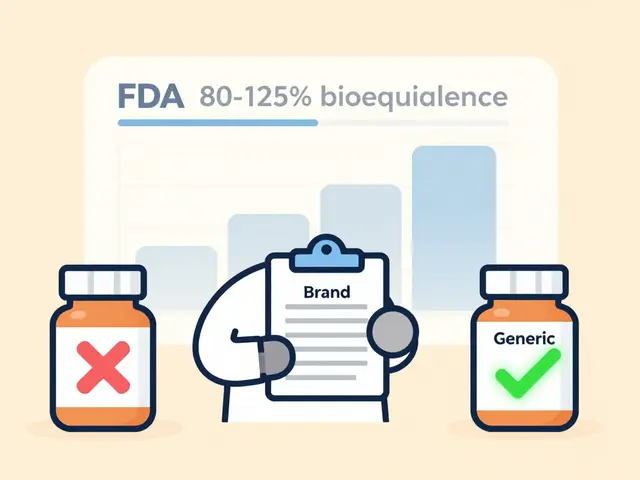
- "The FDA requires generics to show bioequivalence within 80-125% of the brand’s absorption. Real-world data shows 98.7% of approved generics fall within 95-105%-that’s nearly identical."
- "This study of 12.7 million patients found that switching to generics improved adherence by 12.4%. That means fewer hospital visits."
- "This specific generic has the same excipients as the brand. No dye, no lactose. I checked the manufacturer’s specs."
Pharmacists who use specific numbers, cite studies, and reference the Orange Book get a 25% higher acceptance rate than those who just say, "It’s cheaper."
The Right Way to Communicate: Structure Matters
Random calls or vague messages don’t work. Prescribers are busy. They need clarity.
The American Society of Health-System Pharmacists (ASHP) recommends a four-step approach:
- Contact within 24 hours. Don’t wait. If you see a high-cost brand prescription for a chronic condition, act fast.
- Reference the Orange Book rating. Say: "The generic for metformin is rated AB1-therapeutically equivalent."
- Include cost data. "This generic costs $4.50 versus $112 for the brand."
- Document everything. Note the date, method (phone, secure message), who you spoke to, and the outcome.
Pharmacies using this method saw prescriber acceptance jump from 57% to 82%. That’s not luck. That’s structure.
Electronic tools help. Over 87% of prescribers now use Surescripts’ Generic Drug Substitution module. It lets pharmacists send a one-click message with the generic’s NDC, cost, and Orange Book rating directly into the prescriber’s EHR. Communication time drops from 8 minutes to under 3. Documentation goes from 63% complete to nearly 95%.
Barriers Pharmacists Face-And How They Overcome Them
Time is the biggest enemy. The average pharmacist has just 2.3 minutes per prescription to verify, counsel, and communicate. That’s not enough for a long conversation.
Knowledge gaps exist too. One study found 41.7% of pharmacists felt unsure about explaining modified-release generics or complex formulations like transdermal patches.
So how do they keep up?
- The FDA offers free quarterly webinars and "Orange Book Live" Q&As-over 12,000 pharmacists attended in 2022.
- Chain pharmacies now use AI tools like PharmAI’s Generic Substitution Assistant. These tools scan the prescription, pull the Orange Book rating, compare cost, and suggest a message. Accuracy jumped from 76% to 94%.
- Many pharmacists keep a printed cheat sheet: "NTI drugs? Call. Allergies? Check. DAW? Respect. Orange Book? Always check."

Documentation: It’s Not Optional
If you don’t document it, it didn’t happen.
CMS requires pharmacies to record every generic substitution in Medicare Part D claims. In 2023, pharmacies using EHR-integrated systems hit 98.7% compliance. Those using paper logs? Only 76.4%.
The AMA and APhA agree on what to document:
- Date and time of communication
- Method used (phone, secure message, fax)
- Prescriber’s name and credentials
- Generic product dispensed (NDC, manufacturer)
- Reason for substitution (cost, availability, therapeutic equivalence)
- Prescriber’s response (approved, declined, requested follow-up)
Pharmacies that followed these standards saw 27.5% fewer medication errors and 18.3% higher patient satisfaction. Why? Because when a patient says, "My pill looks different," the pharmacist can pull up the record and say, "We called Dr. Lee. You’re on the same drug. Here’s the note."
The Future: Value-Based Care and New Tools
Generic substitution is no longer just a cost-saving tactic-it’s part of value-based care. Sixty-three percent of accountable care organizations (ACOs) now include pharmacist-led generic optimization in their quality metrics.
The 2022 Inflation Reduction Act, effective January 2025, expands Medicare Part D’s medication therapy management (MTM) services. That means pharmacists will be paid to review prescriptions, recommend generics, and follow up with prescribers-not just fill them.
And the FDA is preparing a major update to the Orange Book digital platform in 2024. It will include real-world data on how generics perform in actual patients-not just lab tests. That’s a game-changer.
Meanwhile, the CDC is launching the Generic Medication Safety Network in late 2024. It will give pharmacists near-real-time alerts if a generic brand has a spike in adverse events compared to its brand-name counterpart.
Pharmacists aren’t just dispensers anymore. They’re clinical partners. And when they communicate clearly, accurately, and respectfully with prescribers, they don’t just save money-they save lives.
Can pharmacists substitute generics without the prescriber’s permission?
In 49 states, pharmacists can substitute a generic for a brand-name drug unless the prescriber writes "dispense as written" or "do not substitute." In 17 states, they must also get the patient’s consent. Five states have formularies that limit substitution to specific generic products. Always check your state’s pharmacy board rules.
Are generic drugs really as safe and effective as brand-name drugs?
Yes. The FDA requires generics to prove bioequivalence: their absorption rate must fall within 80-125% of the brand. Real-world data shows 98.7% of approved generics stay within 95-105%, meaning they behave almost identically in the body. Studies of over 12 million patients show no difference in clinical outcomes between generics and brands for most conditions.
What are narrow therapeutic index (NTI) drugs, and why do they need special attention?
NTI drugs have a very small window between an effective dose and a toxic one. Examples include warfarin, levothyroxine, phenytoin, and cyclosporine. Even small changes in blood levels can cause serious side effects or treatment failure. While generics for these drugs meet FDA standards, many prescribers prefer to avoid switching. Pharmacists should always consult the prescriber before substituting NTI drugs.
Why do some patients say a generic doesn’t work the same?
Sometimes it’s psychological-patients associate the brand’s look or packaging with effectiveness. Other times, it’s real: inactive ingredients (like dyes or fillers) can cause allergic reactions or affect absorption in sensitive patients. Rarely, formulation differences in complex drugs like inhalers or extended-release tablets may cause subtle changes. Pharmacists should investigate patient reports, check the Orange Book, and contact the prescriber if needed.
How can pharmacists improve prescriber acceptance of generic recommendations?
Use structured communication: contact within 24 hours, cite the Orange Book rating, provide cost data, and document the exchange. Prescribers respond better to specific evidence-like bioequivalence percentages or adherence improvement stats-than general statements. Using EHR-integrated tools like Surescripts cuts response time and increases documentation accuracy, leading to higher acceptance rates.



Akshaya Gandra _ Student - EastCaryMS
January 6, 2026 AT 02:59saurabh singh
January 6, 2026 AT 19:01Dee Humprey
January 8, 2026 AT 14:08Joseph Snow
January 9, 2026 AT 02:07melissa cucic
January 10, 2026 AT 20:10Shanna Sung
January 11, 2026 AT 16:21Mandy Kowitz
January 13, 2026 AT 11:49Jacob Milano
January 13, 2026 AT 15:36Oluwapelumi Yakubu
January 14, 2026 AT 00:49Terri Gladden
January 15, 2026 AT 04:29Justin Lowans
January 16, 2026 AT 17:52Jennifer Glass
January 17, 2026 AT 03:22