More than 9 in 10 prescriptions filled in the U.S. today are for generic drugs. Yet, many clinicians still hesitate to prescribe them-sometimes because they don’t fully understand what makes a generic safe and effective. This isn’t about cost-cutting alone. It’s about trust. When doctors don’t believe in generics, patients don’t either. And that leads to missed doses, worse outcomes, and higher long-term costs.
Why Clinicians Still Doubt Generics
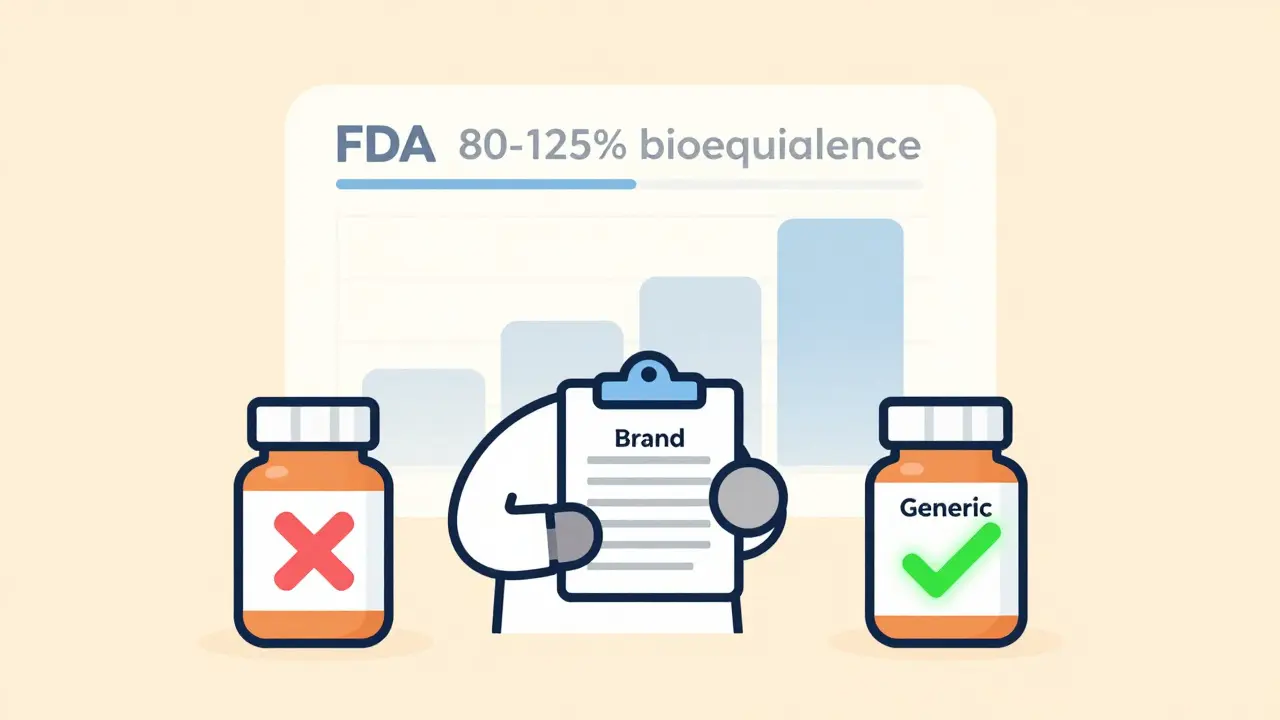
Despite decades of evidence, misconceptions about generic drugs are widespread. A 2020 survey of over 1,200 prescribers found that 45% thought generics had to contain the same inactive ingredients as brand-name drugs. They don’t. Inactive ingredients like fillers and dyes can differ-so long as they don’t affect absorption or safety. Another 38% believed generic manufacturers follow lower quality standards. They don’t. The FDA inspects generic drug facilities just as rigorously as brand-name ones. And 27% wrongly thought bioequivalence meant generics could have up to 25% less active ingredient. The truth? The FDA requires generics to deliver the same amount of active drug into the bloodstream, within a tight 80-125% range of the brand-name version.The Science Behind Generic Approval
Every generic drug must prove bioequivalence through clinical studies. The FDA measures two key values: AUC (how much drug enters the bloodstream over time) and Cmax (how fast it gets there). The 90% confidence interval for both must fall between 80% and 125% of the brand-name drug. That’s not a guess. It’s a statistically validated standard. If a generic doesn’t meet this, it’s rejected. The FDA’s Orange Book lists approved generics and their therapeutic equivalence ratings. An "A" rating means the drug is interchangeable with the brand. A "B" rating means it’s not. Most generics are "A" rated.What Happens When Providers Don’t Understand Generics
When clinicians use brand names in conversations-"Take Lopressor twice daily" instead of "metoprolol"-they unintentionally signal doubt. A resident in Texas described nearly prescribing two doses of metoprolol because her attending said "Lopressor" without mentioning it was the same as the generic she’d already ordered. That kind of confusion isn’t rare. A 2019 national survey found 62% of physicians still default to brand names when prescribing, even when generics are available. This isn’t just outdated practice. It fuels patient anxiety. Studies show patients are more likely to report side effects when told they’re taking a "generic," even if the drug is identical. Harvard researchers found a 18% drop in reported side effects when providers explicitly said, "This generic is just as effective as the brand."Education That Works
Passive materials-flyers, PDFs, webinars-don’t cut it. A 2021 JAMA study showed clinicians who received interactive, case-based training retained 42% more knowledge six months later than those who just read fact sheets. The best programs simulate real patient encounters. For example, the FDA’s new VR training lets prescribers practice explaining generics to skeptical patients. One scenario: a 68-year-old with hypertension who refuses a generic because "it won’t work as well." The trainee learns to respond with data: "The FDA requires this generic to release the same amount of medicine into your blood as the brand. We’ve tested it. It works the same. And it saves you $40 a month."
Where Generics Make the Biggest Difference
Not all drugs are equal in how much they benefit from generic use. Psychiatric medications are a prime example. Patients on antidepressants or antipsychotics often stop taking them because of side effects or cost. When providers confidently prescribe generics, adherence jumps. One study showed patients were 35% more likely to start therapy with a generic than a brand-name version. Chronic conditions like diabetes, high blood pressure, and high cholesterol are next. A 2021 UCSF program reduced brand-name statin prescriptions by 37% after training providers to routinely offer generics. For these patients, cost is the biggest barrier. A $5 generic instead of a $150 brand can mean the difference between taking the drug daily or skipping doses.Barriers to Better Education
Time is the biggest obstacle. Eighty-nine percent of physicians say they don’t have enough time during visits to explain generics. That’s why the most successful programs embed education into clinical workflows. Some EHR systems now show a pop-up when a prescriber selects a brand-name drug: "A generic is available. Would you like to switch?" Others use AI to flag low-generic-prescribing doctors and send personalized learning modules. A 2024 pilot by UnitedHealthcare used this approach and boosted generic prescribing by 28% in just four months.What Clinicians Need to Know
You don’t need to be a pharmacologist to prescribe generics confidently. But you do need to understand a few key facts:- Generics must have the same active ingredient, strength, dosage form, and route of administration as the brand.
- The FDA requires bioequivalence testing with strict 80-125% limits on absorption.
- Inactive ingredients can differ and do not affect safety or efficacy.
- The Orange Book is your go-to source for therapeutic equivalence ratings.
- Thirty-four states allow pharmacists to substitute generics without asking you-unless you write "dispense as written."
- Biosimilars are not generics. They’re complex biologic drugs with no exact copy. Don’t confuse them with small-molecule generics like metformin or lisinopril.

Where to Start
The FDA offers free, evidence-based tools for prescribers. Download the Generic Drug Facts Handout and the Generic Drugs and Health Equity Handout. Both are clear, concise, and ready to use in patient conversations. The Generic Pharmaceutical Association (GPhA) also has short online modules that count toward CME credits. If your hospital or health system doesn’t offer training, ask for it. In 2023, 68 of the 100 largest U.S. health systems made generic education mandatory for new hires. You don’t have to wait for them to catch up.The Bottom Line
Prescribing generics isn’t just about saving money. It’s about saving lives. Patients who take their medications as prescribed live longer, have fewer hospital visits, and report better quality of life. And the strongest predictor of whether a patient will stick with a generic? Whether their doctor says, "This is just as good as the brand."Frequently Asked Questions
Are generic drugs really as effective as brand-name drugs?
Yes. The FDA requires generics to have the same active ingredient, strength, dosage form, and route of administration as the brand-name drug. They must also prove bioequivalence-meaning they deliver the same amount of medicine into the bloodstream at the same rate. Studies show generics work just as well in treating conditions like high blood pressure, depression, and diabetes. The only difference is cost.
Why do some doctors still prefer brand-name drugs?
Many doctors were trained during a time when generics were less common, and brand-name marketing was aggressive. Some still believe outdated myths-that generics are made in lower-quality factories or have different active ingredients. Others worry about patient complaints if a drug doesn’t seem to work as well, even though the difference is often psychological. Education and direct experience with generics can change these views.
Can pharmacists substitute generics without my permission?
In 34 states, yes. Pharmacists can swap a brand-name drug for a generic unless you write "dispense as written" on the prescription. In 16 states, you must explicitly block substitution. In 19 states, substitution is allowed without any notation from you. Check your state’s laws, but remember: if the generic is rated "A" in the FDA’s Orange Book, it’s safe to substitute.
What’s the difference between a generic and a biosimilar?
Generics are exact copies of small-molecule drugs, like metformin or atorvastatin. Biosimilars are highly similar but not identical copies of complex biologic drugs, like insulin or Humira. They’re made from living cells, so they can’t be copied exactly. Biosimilars require more testing than generics, and they’re not interchangeable by default. Only a few have been approved as interchangeable. Don’t assume a biosimilar is a generic-they’re different categories.
How can I learn more about generics without spending hours?
Start with the FDA’s free prescriber toolkit. It includes a 2-page handout with the key facts about generics, plus sample scripts for talking to patients. The Generic Pharmaceutical Association offers 15-minute online modules that count toward CME credits. Many hospitals now have EHR prompts that remind you when a generic is available. You don’t need a full course-just a few minutes a month to stay updated.
Will prescribing generics hurt my patient relationships?
The opposite. Patients appreciate cost savings and transparency. When you explain that a generic is just as effective and say, "I prescribe this to my own family," trust builds. One study found patients were 3.2 times more likely to take a generic if their doctor endorsed it. The real risk to relationships comes from not explaining the choice-or worse, implying the generic is second-rate.


Lana Kabulova
January 23, 2026 AT 09:53I used to think generics were sketchy until my dad’s blood pressure meds switched from brand to generic-and his BP stabilized, and he saved $200/month. No side effects, no ‘weird vibe.’ Just… cheaper, same pill. Why are we still pretending otherwise?
Chiraghuddin Qureshi
January 24, 2026 AT 13:50Bro, in India, generics are the ONLY option for most people 😅. We don’t have luxury of brand names. And guess what? We live longer than you think. 🇮🇳💊 The science is solid. Stop overthinking. The FDA isn’t lying. 🤝
Patrick Roth
January 26, 2026 AT 11:05Oh please. You say the FDA inspects generics the same way? That’s a myth. Ever seen the inspection reports? Most are done by third-party contractors with zero oversight. And don’t get me started on the ‘80-125% bioequivalence’ loophole-that’s not science, it’s a loophole designed to let cheap imports through. I’ve seen generics fail in clinical practice. You’re just repeating marketing fluff.
Lauren Wall
January 27, 2026 AT 04:02Doctors who still say ‘Lopressor’ are part of the problem. End of story.
Jasmine Bryant
January 28, 2026 AT 03:26Just wanted to add-when I worked in a rural clinic, we switched everyone to generics for statins and metformin. Adherence jumped from 52% to 81% in 3 months. Patients didn’t even notice the difference. The real issue? Doctors don’t talk to patients about it. They just prescribe. And then wonder why people stop taking meds. Also, typo: ‘dispense as written’ not ‘dispense as writen’ 😅
Liberty C
January 28, 2026 AT 10:18How utterly predictable. Another feel-good, corporate-funded pamphlet masquerading as ‘evidence.’ You cite the FDA like it’s some infallible oracle-when in reality, it’s a revolving door of ex-pharma executives. And let’s not pretend this is about ‘saving lives.’ It’s about squeezing every penny out of the system while patients get the leftovers. I’ve seen patients on generics who developed unexplained rashes, fatigue, and anxiety-only to feel better the moment they switched back. Coincidence? Or the quiet cost of ‘bioequivalence’?
And don’t give me that ‘35% more likely to start therapy’ nonsense. That’s not adherence-that’s resignation. People take it because they can’t afford the brand, not because they trust it. And you call that progress? Pathetic.
The real villain isn’t the doctor who says ‘Lopressor.’ It’s the entire system that forces patients to choose between health and rent.
shivani acharya
January 29, 2026 AT 04:56Ohhh so now the FDA is the saint of pharmaceuticals? 😂 Tell me, why did 37% of generic metformin batches in 2021 have NDMA contamination? Why did the FDA quietly recall 10+ batches last year? Why do 80% of generic pills come from China and India where inspectors are bribed? You think they care about your ‘80-125%’? They care about profit. And you’re just a pawn in their game. You think your ‘educational modules’ are going to fix this? No. The system is rigged. The pills are made in the same factories that make rat poison. You just don’t want to see it.
And don’t even get me started on the VR training. That’s not education-that’s propaganda. They’re training doctors to lie to patients while smiling. ‘This is just as good’-sure, if you ignore the 30% of people who report worse side effects. But hey, at least it’s cheaper, right? 😘
Daphne Mallari - Tolentino
January 30, 2026 AT 21:15While the intention behind this piece is commendable, its tone is overly reductive and fails to acknowledge the nuanced pharmacokinetic variability that exists even within the FDA’s acceptable bioequivalence parameters. The assumption that all A-rated generics are clinically interchangeable is, in many cases, empirically unsound. Furthermore, the suggestion that clinicians merely need to ‘say the right thing’ to overcome patient skepticism ignores the deep-seated cultural and psychological factors at play. This is not a matter of education alone-it is a systemic failure of trust, communication, and regulatory transparency.
Neil Ellis
February 1, 2026 AT 18:17I love this. Seriously. I used to be skeptical too-until I saw my 72-year-old aunt go from skipping her blood pressure meds to taking them daily because she could afford the generic. She told me, ‘I didn’t think it’d work, but I figured, why not?’ And guess what? Her numbers improved. That’s the magic. Not the pill. The trust. When we say, ‘This is just as good,’ we’re not just prescribing medicine-we’re giving people hope. And that’s worth more than any brand name.
Also, shoutout to the VR training. I tried it. Felt like I was in a doctor’s office in 2050. 🤖❤️
Gerard Jordan
February 3, 2026 AT 00:41Just wanted to say-this is the kind of post that actually changes practice. I’m a resident, and after reading this, I switched my whole script from ‘Lopressor’ to ‘metoprolol’ and added, ‘This is the same as the brand, but way cheaper.’ One patient cried. Said she’d been skipping doses for months. She started taking it the next day. I didn’t save her money-I saved her life. Thank you.