When you pick up a prescription, you might notice your pill looks different this time - maybe it’s a different color, shape, or has new letters stamped on it. That’s not a mistake. It’s likely a different generic manufacturer. Generic drugs are just as safe and effective as brand-name versions, but they often look completely different. Understanding how to compare generic manufacturers and pill appearance helps you avoid confusion, catch errors, and make smarter choices about your meds.
Why Generic Pills Look Different
Generic drugs must contain the same active ingredient, strength, and dosage form as the brand-name version. That’s the law. But the FDA doesn’t require them to look the same. Why? Because brand-name companies hold trademarks on pill shapes, colors, and imprints. To avoid copyright issues, generic makers have to make their pills visually distinct.This means a 10mg lisinopril tablet from Teva might be white and oval, while one from Mylan is blue and round. Both work the same. Both meet FDA standards. But if you don’t know what to look for, you might think you got the wrong medicine.
According to a 2020 study in Nature Scientific Reports, 78% of generic drugs differ in color, 65% in shape, and 42% in size from their brand-name counterparts. These aren’t minor tweaks - they’re major visual changes. And they happen every time a new manufacturer enters the market.
How to Tell If Two Generics Are the Same
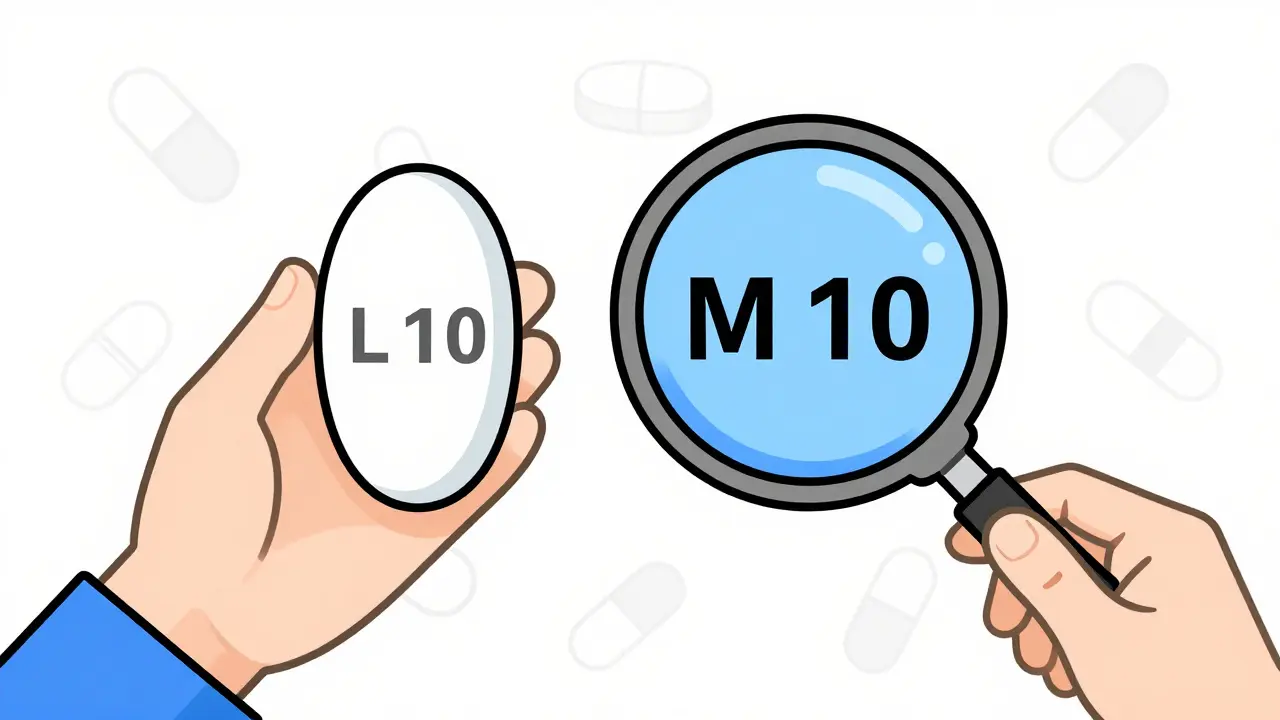
The key is not to rely on looks. Instead, use three reliable identifiers:- The imprint - The letters or numbers stamped on the pill. This is required by federal law (Federal Food, Drug, and Cosmetic Act amendments, 1970). Every prescription pill must have a unique imprint. A pill marked “L 10” is the same drug whether it’s blue, white, or green.
- The National Drug Code (NDC) - A 11-digit number on the pill bottle that identifies the exact manufacturer, drug, and strength. You can look it up in the FDA’s NDC Directory. Two pills with the same NDC are identical, even if they look different.
- The drug name and strength - Always check the label. If it says “Lisinopril 10 mg,” that’s what’s inside. The color doesn’t change the chemistry.
Pharmacists use these three details to verify substitutions. Patients should too. Don’t panic if the pill looks different. Check the imprint and the NDC. If they match what you’ve taken before, you’re fine.
How to Identify Pills by Appearance
If you don’t have the bottle handy - say, you’re traveling or your pill organizer is messy - you can still identify pills using visual clues. The FDA requires all prescription pills to have:- A unique imprint (letters, numbers, or symbols)
- A consistent shape (round, oval, capsule, etc.)
- A specific color (even if it changes between manufacturers)
- A size (measured in millimeters)
Use free tools like Drugs.com Pill Identifier (used by over 12 million people monthly). Just enter the color, shape, and imprint. It will show you all matching drugs - including which manufacturers make them. This is especially helpful if you’re switching between pharmacies or refill sources.
For example, if your 5mg hydrocodone pill used to be white and oval with “V 3601” on it, and now it’s yellow and round with “M 367,” Drugs.com will tell you both are valid versions of the same drug. One is made by Mallinckrodt, the other by Mylan. No change in effectiveness.
What About Quality Differences Between Manufacturers?
Not all generic manufacturers are the same. Some are big, well-established companies. Others are small, low-cost producers. The FDA holds all of them to the same bioequivalence standards: the generic must deliver the same amount of active ingredient into your bloodstream as the brand, within a 80-125% range. That’s a tight window - and it’s been proven to work.A landmark FDA study of over 2,000 bioequivalence tests found that, on average, generics differed from brand-name drugs by only 3.5% in absorption. That’s less variation than you’d see between two batches of the same brand-name drug.
But there are exceptions. For drugs with a narrow therapeutic index (NTIDs) - like warfarin, levothyroxine, or lithium - the acceptable range is tighter: 90-111%. Even small changes in blood levels can cause problems. That’s why doctors often recommend sticking with the same generic manufacturer for these drugs.
Major manufacturers like Teva, Mylan (now Viatris), Sandoz, and Hikma have reputations for consistency. Teva alone makes over 1,800 generic drugs and holds 9% of the U.S. market. They’ve been around since 1901. Smaller manufacturers may cut corners on packaging or testing. While still FDA-approved, they’re more likely to be involved in supply shortages.
The FDA’s 2024 Drug Shortage Report found that 67% of active shortages involve generic medications. That’s not because generics are unsafe - it’s because some manufacturers can’t keep up with demand or have production issues. If you notice your pill suddenly looks different and you’ve had side effects since the switch, talk to your pharmacist.
When Appearance Changes Might Matter
Most of the time, a change in pill appearance means nothing. But there are three red flags:- You’re on a narrow therapeutic index drug. If you take levothyroxine for hypothyroidism or warfarin for blood thinning, even small changes in absorption can cause symptoms like fatigue, palpitations, or abnormal bleeding. If your pill looks different, ask your pharmacist if the manufacturer changed.
- You notice new side effects. If you start feeling dizzy, nauseous, or unusually tired after a pill change - especially if you’ve been stable for months - it might be worth switching back. The inactive ingredients (fillers, dyes, binders) can affect how your body absorbs the drug. A 2012 case study linked a Lamotrigine formulation change to unexplained toxicity in epilepsy patients.
- The pill has no imprint. All FDA-approved prescription pills must have an imprint. If yours doesn’t, it could be counterfeit, a non-prescription supplement, or a foreign product. Never take it.
Patients who switch generics often report feeling “off.” A 2022 Consumer Reports survey found that 41% of users were concerned about appearance changes. But 68% of those who actually switched said they felt no difference. The anxiety is real - but the risk is low, unless you’re on a high-risk drug.
What Pharmacists and Doctors Recommend
The American Medical Association’s 2023 policy says: “Generic substitution is safe for most patients. For narrow therapeutic index drugs, maintain the same manufacturer unless medically necessary.”Pharmacists are trained to catch these issues. If you’re on warfarin, they’ll likely use a “Dispense As Written” (DAW-1) code to prevent substitutions. If you’re on a common drug like metformin, they may switch manufacturers to save you money.
They’re also trained to explain this to you. Ask: “Is this the same drug I was on before?” or “Has the manufacturer changed?” Most will show you the NDC code or pull up the pill image on their computer.
Healthcare providers now use the “5 Rights” framework to prevent errors:
- Right drug
- Right dose
- Right time
- Right route
- Right patient
Appearance isn’t part of the 5 Rights - because it shouldn’t be. The pill’s identity comes from its name, strength, and imprint - not its color.
How to Stay in Control
Here’s what you can do today:- Keep a list of your meds, including the imprint and NDC code from the bottle.
- Use Drugs.com or another pill identifier app to check new pills before taking them.
- Ask your pharmacist if the manufacturer changed when you refill.
- Never stop or switch your medicine without talking to your doctor - especially if you’re on an NTID drug.
- Report suspicious pills to the FDA’s MedWatch program if a pill has no imprint, looks fake, or causes unexpected side effects.
Generic drugs save Americans an average of $265 per month compared to brand-name versions. That’s real money. And they work. But you have to know how to read them.
The next time your pill looks different, don’t assume something’s wrong. Check the imprint. Look up the NDC. Use a reliable tool. And if you’re on a high-risk drug - don’t hesitate to ask for the same manufacturer. You’re not being difficult. You’re being smart.
Are generic drugs as effective as brand-name drugs?
Yes, for the vast majority of medications. The FDA requires generic drugs to have the same active ingredient, strength, dosage form, and route of administration as the brand-name version. They must also prove bioequivalence - meaning they deliver the same amount of drug into your bloodstream within a strict 80-125% range. Studies show the average difference in absorption between generics and brand-name drugs is only 3.5%, which is less than the variation seen between two batches of the same brand. For most people, generics work just as well.
Why do generic pills look different from brand-name pills?
Brand-name companies hold trademarks on the color, shape, and imprint of their pills. To avoid violating those trademarks, generic manufacturers must make their versions visually different. This is required by law. So even if two pills contain the exact same drug and dose, one might be blue and oval, while the other is white and round. The difference is only cosmetic - not functional.
Can I switch between different generic manufacturers?
For most medications, yes. But for drugs with a narrow therapeutic index - like warfarin, levothyroxine, lithium, or phenytoin - switching between manufacturers can pose risks. Even small changes in how the drug is absorbed can lead to under- or over-treatment. In these cases, your doctor or pharmacist may recommend sticking with the same generic manufacturer. Always check with them before switching.
How do I know if my pill is from a reputable manufacturer?
All FDA-approved generic manufacturers must meet the same quality standards. But some are more reliable due to scale and history. Major companies like Teva, Mylan (Viatris), Sandoz, and Hikma have been producing generics for decades and have fewer supply issues. You can check the manufacturer name on your prescription bottle or use the NDC code to look up the company in the FDA’s NDC Directory. Avoid pills with no imprint - those are not FDA-approved prescription drugs.
What should I do if my pill looks different and I feel worse?
If you notice new side effects - like dizziness, nausea, unusual fatigue, or changes in heart rate - after switching to a different generic, contact your pharmacist or doctor. Ask if the manufacturer changed. If you’re on a narrow therapeutic index drug, ask to return to your previous version. Don’t assume it’s all in your head. The FDA has documented cases where formulation changes caused unexpected reactions. Your health comes first.


Michaela Jorstad
February 19, 2026 AT 16:17Thank you for this! I’ve been switching generics for years and always second-guessed myself. Now I know to check the imprint-L 10 for lisinopril, M 367 for hydrocodone. I keep a note in my phone with pics of my pills. It’s weird how much peace of mind that gives you.