When two or more drugs are combined into a single pill or formulation, the goal is simple: better results with fewer pills. But what happens when you switch from one brand to another, or from a brand to a generic, and the doses aren’t exactly the same? This is where therapeutic equivalence becomes critical-especially in combination products.
What Therapeutic Equivalence Really Means
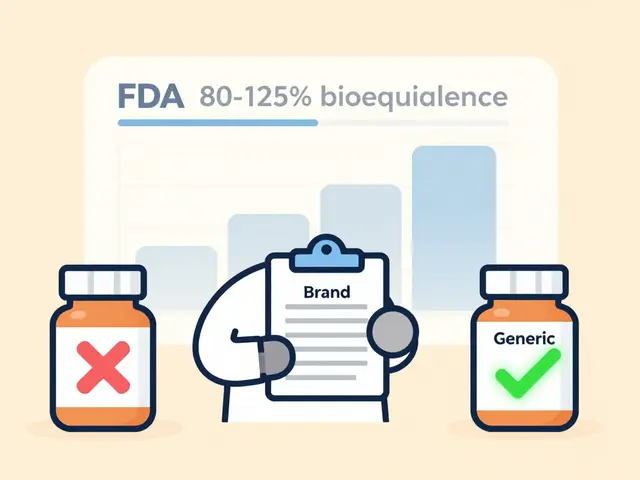
Therapeutic equivalence isn’t just about two drugs having the same name. It’s about whether they produce the same clinical effect and safety profile in real patients. The U.S. Food and Drug Administration (FDA) defines it strictly: two products must contain identical active ingredients, in the same strength, dosage form, and route of administration. That’s it. No wiggle room. If you’re switching from brand-name Advair Diskus to a generic version of fluticasone/salmeterol, the active ingredients must match exactly-and the FDA’s Orange Book confirms whether they do. The Orange Book, first published in 1980, now lists over 14,000 drug products with therapeutic equivalence ratings. About 95% of them are rated "A," meaning they’re considered interchangeable. That’s a huge win for cost savings. Generic drugs saved the U.S. healthcare system $1.7 trillion over the last decade. But here’s the catch: not all combinations are created equal.Why Dose Equivalence Gets Complicated
Combination products like amlodipine/benazepril or tramadol/acetaminophen don’t just add up like numbers. Their components often interact. One drug might boost the effect of the other. That’s called synergy. For example, tramadol and acetaminophen together reduce pain more effectively than either drug alone at the same total dose. That means you can’t just swap one combination for another based on total milligrams-you have to account for how each component behaves. This is where dose equivalence calculations get technical. Researchers use formulas like beq(a) = CBγ(1+CAa)−1 to figure out how much of one drug equals the effect of another, based on their maximum efficacy. In one study, sirolimus reduced vascular cell growth by 69.8%, while topotecan hit 88.9%. To match their effects, you’d need to adjust the doses accordingly. That’s not something you can eyeball. It requires pharmacokinetic modeling.The FDA’s TE Code System: A, B, and What They Mean
The FDA uses a letter code system to tell pharmacists and doctors whether a product is interchangeable. An "A" rating means the product is therapeutically equivalent to the reference drug. A "B" rating means there’s uncertainty-maybe the bioavailability isn’t proven, or the formulation differs in a way that could affect absorption. For combination products, things get messier. Some combinations are approved under a 505(b)(2) pathway, which allows modifications to existing drugs. If those changes affect how the body absorbs one component, the product might get a "B" rating-even if the active ingredients look identical on paper. For example, if a generic version of a combination drug uses a different filler or coating, and that changes how quickly the drug dissolves, it might not be considered equivalent.Narrow Therapeutic Index Drugs: When Small Changes Matter
Some drugs have a razor-thin line between working and causing harm. These are called narrow therapeutic index (NTI) drugs. Think warfarin, levothyroxine, phenytoin. With these, even a 5% difference in blood levels can lead to serious side effects or treatment failure. The FDA requires stricter bioequivalence standards for NTI drugs: 90-111% instead of the usual 80-125%. But even that doesn’t always prevent problems. A 2018 study found that 12% of patients switching between different generic versions of levothyroxine experienced changes in thyroid hormone levels-despite meeting FDA standards. In combination products, this risk multiplies. If one component is an NTI drug, like levothyroxine paired with a calcium supplement, the margin for error shrinks even further.Real-World Mistakes and Near Misses
Behind every statistic is a real patient. A pharmacist in Texas reported three dosing errors in six months from switching between different strengths of amlodipine/benazepril combinations. One patient got a 10/20mg version instead of 5/20mg-thinking they were interchangeable. The result? A sudden drop in blood pressure. Another case involved a patient switched from brand-name Vytorin (ezetimibe/simvastatin) to a generic. LDL cholesterol went up 15%. The generic had the same active ingredients and met FDA bioequivalence standards. But the inactive ingredients-like binders and coatings-changed slightly. That small difference affected how the simvastatin was absorbed. The FDA’s Adverse Event Reporting System recorded 247 incidents in 2022 related to dose conversion errors in combination products. Nearly 40% involved cardiovascular drugs. Another 30% involved psychiatric combinations, where even small fluctuations can trigger anxiety, seizures, or mood swings.How to Manage Therapeutic Equivalence Safely
There’s no magic bullet, but there are proven steps:- Check the Orange Book. Always verify the TE code before substituting. Don’t assume two drugs with the same name are interchangeable.
- Don’t swap NTI combinations lightly. If one component is an NTI drug, treat the whole combination as high-risk. Monitor labs and symptoms closely after any switch.
- Use standardized conversion tables. The Institute for Safe Medication Practices offers printable guides for common combinations. Keep them in your pharmacy or clinic.
- Scan barcodes. Barcode scanning at the point of dispensing catches 90% of dosage errors. If your system doesn’t have it, push for it.
- Monitor for 72 hours after switching. Especially for cardiac, psychiatric, or anticoagulant combinations. Call the patient. Ask how they feel. Check vital signs or lab values if needed.
What’s Changing in 2025
The FDA is moving beyond simple A/B ratings. In early 2023, they released draft guidance for "complex combination products"-those where the dose-response isn’t linear, or where one component affects the other’s metabolism. They’re testing machine learning models to predict which generic versions might cause problems based on formulation differences. Early results show 89% accuracy. There’s also talk of an "A*" rating for combinations that have been proven equivalent across multiple strengths. Right now, a drug might be rated "A" at 5/20mg but not at 10/40mg. That’s confusing. A unified rating system could fix that. And long-term? The Precision Medicine Initiative predicts that by 2030, 30% of therapeutic equivalence decisions will include genetic data. Someone who metabolizes drugs slowly might need a different dose-even if the product is labeled "therapeutically equivalent."Bottom Line: Equivalence Isn’t Always Equal
Therapeutic equivalence is a powerful tool. It saves money, expands access, and keeps patients on their meds. But it’s not a guarantee of identical outcomes. In combination products, especially those with NTI drugs or synergistic effects, the devil is in the details. The best practice? Don’t rely on labels alone. Know your drugs. Know your patients. And when in doubt-don’t substitute without checking.What does an "A" rating mean in the FDA Orange Book?
An "A" rating means the drug product is therapeutically equivalent to the reference listed drug. It contains the same active ingredients, strength, dosage form, and route of administration, and has been shown to be bioequivalent. These products are considered interchangeable by the FDA.
Can generic combination drugs always be substituted for brand-name versions?
Not always. While most combination generics have an "A" rating and can be safely substituted, some-especially those containing narrow therapeutic index drugs or complex formulations-may have subtle differences in inactive ingredients that affect absorption. Always check the Orange Book and monitor patients after switching.
Why are dose equivalents tricky in combination products?
Because the components can interact. One drug may enhance or reduce the effect of the other, making simple additive math inaccurate. For example, tramadol and acetaminophen work better together than separately. Switching to a different combination with the same total milligrams might not give the same pain relief.
What’s the difference between a pharmaceutical equivalent and a therapeutic equivalent?
A pharmaceutical equivalent has the same active ingredients, strength, dosage form, and route of administration. A therapeutic equivalent meets those criteria AND has been proven to produce the same clinical effect and safety profile. All therapeutic equivalents are pharmaceutical equivalents, but not all pharmaceutical equivalents are therapeutic equivalents.
Are there risks when switching between different generic versions of the same combination drug?
Yes. Even if two generics have the same TE code, they may use different inactive ingredients-like binders or coatings-that affect how quickly the drug is absorbed. This is especially risky with narrow therapeutic index drugs. Patients may experience changes in effectiveness or side effects even if the switch is "approved."
How can pharmacists reduce errors with combination products?
Use the FDA Orange Book to verify TE codes, implement barcode scanning, maintain standardized conversion tables, and monitor patients for 72 hours after switching. Educate staff on the unique risks of combination products, especially those with NTI components. Avoid automatic substitutions without clinical review.
What role does the FDA play in therapeutic equivalence for combination products?
The FDA evaluates combination products for bioequivalence and assigns therapeutic equivalence ratings (A or B) in the Orange Book. They set standards for active ingredient matching, dosage form, and bioavailability. For complex or NTI combinations, they require stricter testing. The FDA also updates guidance based on new data, like their 2023 draft on complex combinations.
Is therapeutic equivalence the same in the European Union?
The European Medicines Agency (EMA) follows similar principles but often requires additional in-vivo studies for fixed-dose combinations, especially when components have different absorption rates. While the U.S. relies heavily on bioequivalence data, the EMA may require clinical endpoint data in some cases, making approval more rigorous.



Wendy Edwards
November 27, 2025 AT 02:34Jaspreet Kaur
November 27, 2025 AT 16:27Gina Banh
November 28, 2025 AT 08:10Deirdre Wilson
November 29, 2025 AT 01:12Damon Stangherlin
November 30, 2025 AT 13:12Ryan C
November 30, 2025 AT 22:50Dan Rua
December 1, 2025 AT 15:14Mqondisi Gumede
December 1, 2025 AT 23:08Douglas Fisher
December 2, 2025 AT 07:54Albert Guasch
December 4, 2025 AT 04:17Ginger Henderson
December 6, 2025 AT 01:56