Orange Book TE Code Checker
Check Your Drug's Therapeutic Equivalence Code
Enter a drug name or active ingredient to see its Orange Book TE code and substitution safety information.
Therapeutic Equivalence Code
What This Means for Substitution
Every time you pick up a generic prescription at the pharmacy, there’s a quiet but powerful system behind that decision. It’s not luck. It’s not guesswork. It’s the Orange Book - the FDA’s official guide that tells pharmacists whether a generic drug can safely replace the brand-name version. If you’ve ever wondered why your pharmacist swapped your brand medication for a cheaper version without asking, or why some generics aren’t interchangeable, the answer lies in this document.
What Is the Orange Book?
The Orange Book is the FDA’s official publication titled Approved Drug Products with Therapeutic Equivalence Evaluations. First published in 1980, it was created to implement the Hatch-Waxman Act of 1984, a law designed to balance innovation and affordability in medicine. Its job? To identify which generic drugs are therapeutically equivalent to their brand-name counterparts - meaning they work the same way in the body, with the same safety and effectiveness.It’s not just a list. It’s a decision engine. Every drug entry includes a Therapeutic Equivalence (TE) code - a two-letter rating that tells pharmacists and insurers whether substitution is allowed. The Orange Book is updated every month, and as of September 2023, it covers over 15,000 approved drug products. That includes everything from simple antibiotics to complex inhalers and topical creams.
What Makes a Generic Drug Therapeutically Equivalent?
Not all generics are created equal. For a generic to be rated as therapeutically equivalent, it must pass three strict tests:- Pharmaceutical equivalence: The generic must contain the exact same active ingredient, in the same strength, dosage form (tablet, capsule, injection), and route of administration (oral, topical, etc.) as the brand-name drug. It must also meet the same quality and purity standards.
- Bioequivalence: The generic must be absorbed into the bloodstream at the same rate and to the same extent as the brand. This isn’t just about ingredients - it’s about how the body processes them. The FDA requires clinical studies proving that the amount of drug in your blood over time is within a narrow, acceptable range compared to the original.
- FDA approval: The generic must be manufactured under the same strict quality controls (Current Good Manufacturing Practices) as the brand, and its labeling must be accurate and complete.
If all three are met, the FDA assigns it an A code. If any part fails - even if the active ingredient is the same - it gets a B code. And that makes all the difference.
Understanding TE Codes: A, B, X, and More
The TE code is the key to substitution. Here’s what the letters mean:- AB: The gold standard. The generic is therapeutically equivalent with no known bioequivalence issues. Pharmacists can substitute it freely.
- AN: An aerosol inhaler or nasal spray that’s equivalent. These are complex because delivery devices matter, but the FDA has confirmed the generic works the same way.
- AO: A topical product (like a cream or ointment) that’s equivalent. Again, these are tricky due to skin absorption, but the FDA has approved substitution.
- AX: Not equivalent. The drug is approved, but there’s not enough data to prove bioequivalence. Don’t substitute.
- BC: A drug with potential bioequivalence problems. This often applies to narrow therapeutic index drugs - where small changes in blood levels can cause serious side effects or reduced effectiveness. Substitution is risky without doctor approval.
- BD: Similar to BC, but with documented issues in clinical studies. Avoid substitution unless absolutely necessary.
For example, warfarin (a blood thinner) is a narrow therapeutic index drug. Even if two versions are both labeled “AB,” pharmacists and doctors often stick with the same brand because tiny differences in absorption can increase the risk of clotting or bleeding. The Orange Book flags these cases, but the final call often involves the prescriber.
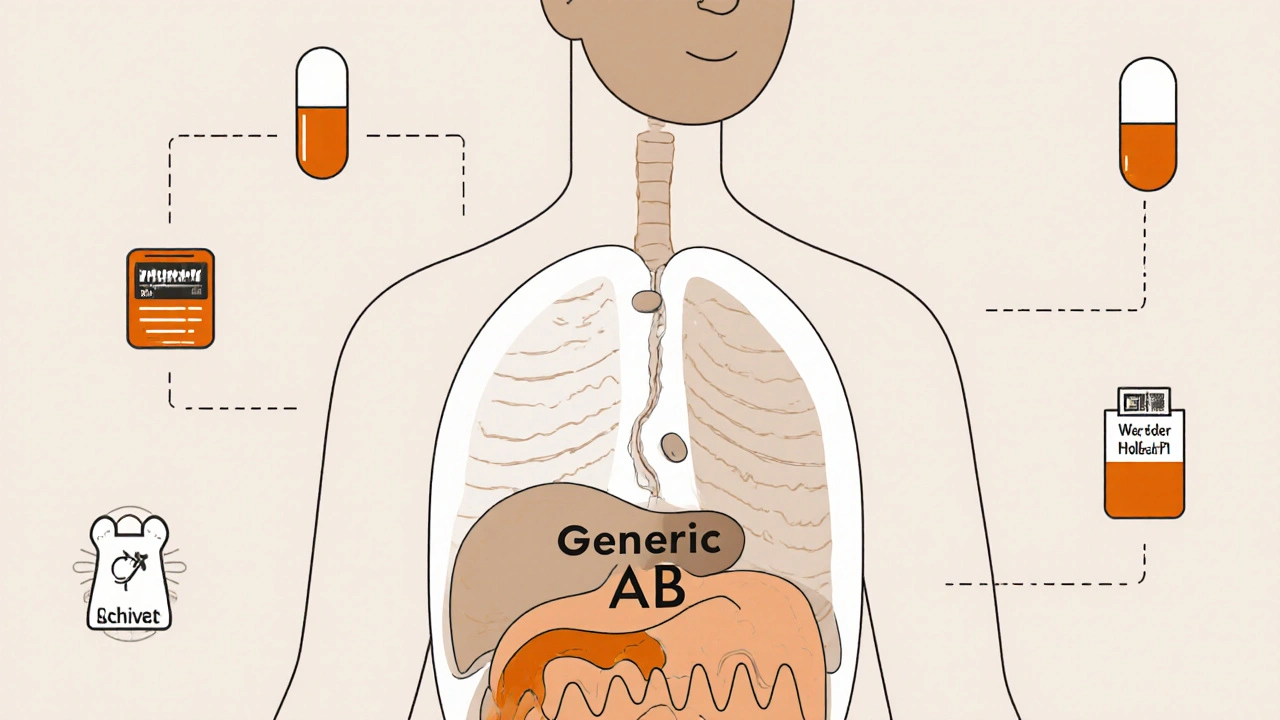
How the Orange Book Impacts Your Healthcare
The impact of the Orange Book goes far beyond the pharmacy counter. Insurance companies, pharmacy benefit managers (PBMs), and state laws all rely on its TE codes to decide what gets covered and what gets substituted automatically.In 2022, 90.7% of all prescriptions filled in the U.S. were for generic drugs - but they made up only 22.8% of total drug spending. That’s because generics cost far less. Over the last decade, the Orange Book helped save the U.S. healthcare system over $1.67 trillion by enabling safe substitution.
But here’s the catch: not everyone understands it. A 2023 survey by Pharmacy Times found that 67% of pharmacists found TE codes “moderately to extremely difficult” to interpret without training. One Walgreens report showed that incorrect TE code use led to $1.2 million in rejected insurance claims in just one quarter. Meanwhile, CVS Health’s automated system, which checks TE codes in real time, cut substitution errors by 63% and saved $47 million annually.
That’s why training matters. The National Community Pharmacists Association offers a 4-hour certification course on Orange Book interpretation. In 2022, over 8,400 pharmacists took it. The FDA offers free online modules, but only 41% of community pharmacists say they feel “very confident” using the system without reference materials.
What’s Not in the Orange Book?
The Orange Book doesn’t cover everything. It excludes drugs approved only for safety, like older medications reviewed under the DESI program (e.g., Donnatal Tablets, Librax Capsules). It also doesn’t include pre-1938 drugs like Phenobarbital Tablets, which were grandfathered in before modern approval standards.It doesn’t compare different drugs that treat the same condition - like morphine vs. meperidine for pain. Those are different chemical compounds with different effects. The Orange Book only compares exact copies - same active ingredient, same delivery method.
And it doesn’t cover biologics - complex drugs made from living cells, like insulin or Humira. Those have their own approval pathway and aren’t listed in the Orange Book. Biosimilars (similar but not identical copies) are tracked separately.

The Future of the Orange Book
The FDA is updating the Orange Book for the digital age. The old print version is gone. Now, it’s a searchable online database, with monthly updates. By mid-2024, the new version will include application numbers, manufacturer names, and drug strengths all in one place - making it easier for pharmacists, prescribers, and even patients to check substitution status.The agency is also refining how it evaluates complex products - like inhalers, injectables, and topical creams - where tiny differences in formulation or delivery can affect outcomes. In 2022, the FDA released new guidance to clarify standards for these drugs, ensuring the Orange Book stays relevant as medicine gets more sophisticated.
But the core mission hasn’t changed: keep safe, affordable drugs accessible. Every time a pharmacist hands you a generic pill instead of the brand, they’re following the Orange Book’s rules. And that system - built on science, not speculation - is why millions of Americans pay less for their meds every day.
What You Should Do
If you’re on a generic medication:- Check your prescription label. If it says “Substitution Permitted,” the pharmacist used the Orange Book to confirm equivalence.
- If you’re switched to a different generic and feel different - worse side effects, less relief - tell your doctor. It could be a bioequivalence issue.
- Ask your pharmacist for the TE code of your drug. If it’s an “AB,” you’re good. If it’s “BC” or “BX,” ask if substitution is safe in your case.
- Don’t assume all generics are the same. Even two “AB” rated drugs might have different inactive ingredients that affect you.
For prescribers: Always check the TE code before writing a prescription. If you don’t want substitution, write “Dispense as Written” or “Do Not Substitute.” That’s your legal right.
What does an AB rating mean in the Orange Book?
An AB rating means the generic drug is therapeutically equivalent to the brand-name drug. It has the same active ingredient, dosage form, strength, and route of administration, and has been proven bioequivalent through FDA-approved testing. Pharmacists can substitute an AB-rated generic without needing the prescriber’s approval.
Can I request a brand-name drug instead of a generic?
Yes. Even if a generic is rated AB and substitution is allowed, you can ask your pharmacist to dispense the brand-name version. You’ll likely pay more, unless your insurance covers it. Your prescriber can also write “Dispense as Written” on the prescription to prevent substitution.
Why are some generics not interchangeable even if they have the same active ingredient?
Two generics can have the same active ingredient but differ in how they’re made - like the coating, filler, or delivery system. For complex drugs like inhalers, topical creams, or injectables, these differences can affect how the drug is absorbed. If the FDA hasn’t confirmed bioequivalence, it assigns a B code (like BC or BX), meaning substitution isn’t recommended.
Is the Orange Book only for prescription drugs?
No. The Orange Book includes both prescription and over-the-counter (OTC) drugs. Part II of the book lists OTC products that aren’t covered under standard monographs. But it doesn’t include dietary supplements, vitamins, or herbal products - only FDA-approved drugs.
How often is the Orange Book updated?
The Orange Book is updated monthly. New generic approvals, withdrawn products, and changes to TE codes are added each month. The FDA releases a new version on the last business day of each month. Pharmacists and pharmacies use this updated data to ensure accurate substitution.
Are biosimilars listed in the Orange Book?
No. Biosimilars - which are similar to complex biologic drugs like Humira or Enbrel - are not included in the Orange Book. They follow a different approval pathway under the Biologics Price Competition and Innovation Act and are listed in a separate FDA database called the Purple Book.
Next Steps for Patients and Providers
If you’re a patient: Use the FDA’s free online Orange Book database to look up your medication. Search by brand name or generic ingredient. Check the TE code. If you’re unsure, ask your pharmacist to explain it. Don’t be afraid to ask questions - your safety depends on it.If you’re a provider: Always verify TE codes before prescribing. Use the FDA’s electronic database, not outdated printouts. When prescribing for drugs with narrow therapeutic indexes - like warfarin, lithium, or phenytoin - consider specifying “Do Not Substitute” unless you’re confident the patient has been stable on the generic.
The Orange Book isn’t perfect. But it’s the most reliable tool we have to ensure that generic drugs are safe, effective, and affordable. And for millions of people, that’s the difference between taking their medicine - and not taking it at all.




Holli Yancey
November 16, 2025 AT 23:51It's wild how something so technical can literally save lives by cutting costs. I never thought about the Orange Book before, but now I see why my insulin generic never gave me the same side effects as the first one I got. It's not magic-it's science.
Jessica Healey
November 18, 2025 AT 07:36my pharmacist switched me to a generic last month and i felt like i was drugged out for a week. turned out it was a BC-rated one. i didn't even know those codes existed until now. thanks for explaining.
Kathryn Ware
November 19, 2025 AT 15:56As a pharmacist for 18 years, I’ve seen the Orange Book transform care. I used to have to flip through printed binders every week-now I just pull up the FDA site on my phone. The monthly updates? Lifesaver. And yes, I’ve had patients cry because they couldn’t afford their brand anymore. The Orange Book lets them breathe again. 💙
Gordon Mcdonough
November 19, 2025 AT 22:17THIS IS WHY AMERICA IS STILL THE BEST!! The FDA doesn't mess around! Other countries let anything pass as generic-here we got science, we got standards, we got AB ratings!! Who needs Europe when we got this??
Elia DOnald Maluleke
November 21, 2025 AT 17:33One might argue that the Orange Book represents the institutionalization of epistemological certainty in pharmacology-a system where bioequivalence is not merely measured, but canonized. The very notion that a pill’s absorption profile can be reduced to a two-letter code speaks volumes about the modern condition: we seek simplicity in complexity, and in doing so, we risk flattening the phenomenology of bodily experience into a spreadsheet. Is therapeutic equivalence, then, an objective truth-or merely the consensus of regulatory aesthetics?
Levi Hobbs
November 21, 2025 AT 19:57Just wanted to say thanks to the OP for laying this out so clearly. I’m a med student and I’ve been confused about TE codes for months. This is the first time I’ve seen it explained without jargon overload. The part about inhalers and topical creams? Eye-opening. I’ll be sharing this with my cohort.
Sridhar Suvarna
November 23, 2025 AT 06:49India’s generic industry feeds the world. But we don’t have an Orange Book. We have trust. And sometimes, that’s not enough. I hope one day our regulators adopt something like this-not to limit access, but to protect it. Knowledge is the real medicine.
kora ortiz
November 24, 2025 AT 10:25Finally someone gets it. I’ve been telling my doctor for years not to let them swap my seizure med. Turns out I was right. AB rating? Good. BC? No thanks. Knowledge is power. Keep spreading this info!
Kelsey Robertson
November 26, 2025 AT 00:23Of course the FDA says it’s safe. But who’s really behind the Orange Book? Big Pharma owns the FDA, right? They let generics in-just enough to keep prices low-but only if they don’t threaten the brand’s profit margins. AB ratings? Maybe just a marketing trick to make you think you’re getting the same thing. I’ve seen people get sicker after switching. Coincidence?
Joseph Townsend
November 26, 2025 AT 02:15Bro. The Orange Book is the unsung hero of American healthcare. Like, imagine if your Netflix algorithm could swap out your favorite show for a knockoff that ‘works the same’-but you start hallucinating after episode 3. That’s what happens when you don’t read the TE code. I now check mine like a Bible. AB = peace. BC = panic.
henry mariono
November 26, 2025 AT 14:40I appreciate the depth here. I used to assume all generics were equal. Now I know better. I’ll be asking my pharmacist for the TE code from now on. No more guessing. Thank you.
Bill Machi
November 28, 2025 AT 09:471.67 trillion saved? That’s a number cooked up by bureaucrats to justify their existence. Meanwhile, people are getting sick from generics that look identical but behave differently. The FDA doesn’t test real-world outcomes-they test lab results. Big difference. And don’t get me started on how PBMs force substitutions to hit quotas. This isn’t science-it’s cost-cutting dressed up as progress.
shubham seth
November 28, 2025 AT 21:51Let’s be real-the Orange Book is a glorified spreadsheet. They rate drugs based on bioequivalence studies that use 24 healthy young men. What about elderly patients? Diabetics? People with liver disease? The system ignores the real world. AB rating means nothing if your body doesn’t process the damn thing the same way. This is why people die on generics. And nobody talks about it.
Joseph Peel
November 30, 2025 AT 10:46For years, I thought generic meant ‘cheaper version.’ Now I know it’s ‘FDA-verified clone.’ The TE codes are the invisible hand of patient safety. This post should be required reading for every high school health class.
Jeremy Hernandez
December 1, 2025 AT 08:30They’re lying. The Orange Book doesn’t exist. It’s all a front. The real system is controlled by a secret FDA-PBM cartel that picks which generics get AB ratings based on bribes. My cousin’s dad died after switching. The FDA buried the report. Google ‘Orange Book coverup’ and you’ll see.