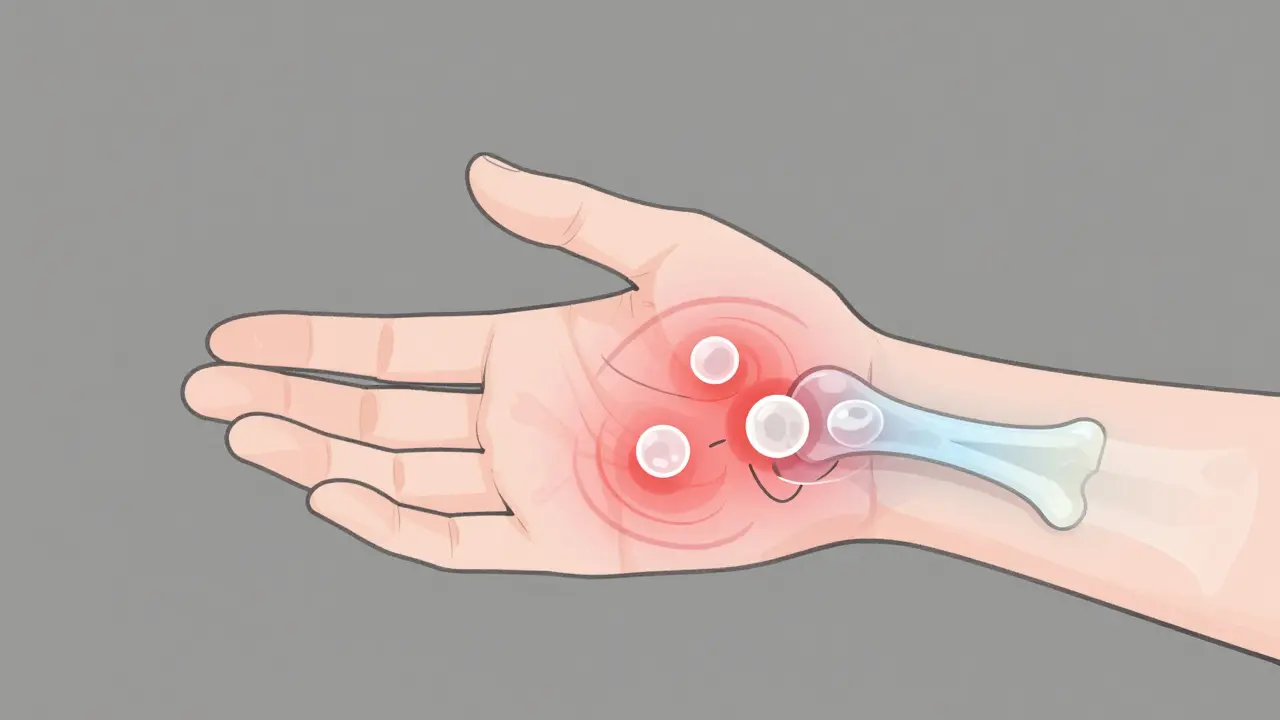
When your immune system turns on your own body, it doesn’t just cause a fever or a runny nose. In rheumatoid arthritis (RA), it attacks the lining of your joints-called the synovium-turning everyday movements into pain. This isn’t just aging or overuse. It’s an autoimmune war happening inside your wrists, fingers, knees, and feet. And if left unchecked, it can destroy cartilage, erode bone, and leave you unable to hold a cup, button a shirt, or walk without discomfort.
What Makes Rheumatoid Arthritis Different from Other Arthritis?
Many people think all arthritis is the same-worn-out joints from years of activity. But osteoarthritis is mechanical wear and tear. Rheumatoid arthritis is a system-wide autoimmune attack. Your immune system, which should protect you from viruses and bacteria, starts producing antibodies that target healthy joint tissue. This triggers inflammation, swelling, and eventually, permanent damage.
RA doesn’t hit just one joint. It usually strikes symmetrically-both wrists, both knees, both ankles. Symptoms creep in slowly, often over weeks or months. Morning stiffness that lasts longer than 45 minutes? That’s a red flag. So is persistent pain and swelling in small joints of the hands and feet. The American College of Rheumatology says if these symptoms last six weeks or more, it’s time to get tested.
Blood tests help confirm it. Rheumatoid factor (RF) and anti-CCP antibodies are markers found in most RA patients. X-rays and MRIs show early signs like soft tissue swelling, then later, bone erosion and narrowing joint spaces. About 10-15% of people with RA also develop Sjögren’s syndrome-dry eyes and dry mouth-because the immune system attacks moisture-producing glands too.
More Than Just Joint Pain: The Systemic Toll of RA
Rheumatoid arthritis isn’t just a joint disease. It’s a whole-body condition. Chronic inflammation doesn’t stay in the hands. It spreads.
You might develop rheumatoid nodules-hard lumps under the skin near elbows or heels. Your lungs can get inflamed, leading to shortness of breath. Blood vessels may become damaged, increasing your risk of heart attack or stroke. Anemia is common, leaving you tired even after a full night’s sleep. Studies show RA patients have a 50% higher risk of cardiovascular disease than the general population.
And it’s not just physical. Living with constant pain, unpredictable flares, and the fear of losing mobility takes a mental toll. One survey of 28,500 people on Reddit’s RA community found 78% reported morning stiffness lasting over an hour. Many described feeling isolated, frustrated, or even depressed. The emotional burden is real-and often overlooked.
How RA Is Treated: From Pain Relief to Stopping the Attack
For decades, treatment focused on easing symptoms: NSAIDs for pain, steroids for quick relief. But those don’t stop the disease. The real goal now is to stop the immune system from destroying your joints before it’s too late.
The first line of defense is usually methotrexate, a traditional DMARD (disease-modifying antirheumatic drug). It’s been used since the 1980s, works for most people, and costs under $50 a month. But about 40-50% of patients don’t respond well enough. That’s where biologic therapies come in.
Biologics are made from living cells and target very specific parts of the immune system. Unlike methotrexate, which broadly suppresses immunity, biologics are like precision missiles. They block the signals that cause inflammation.
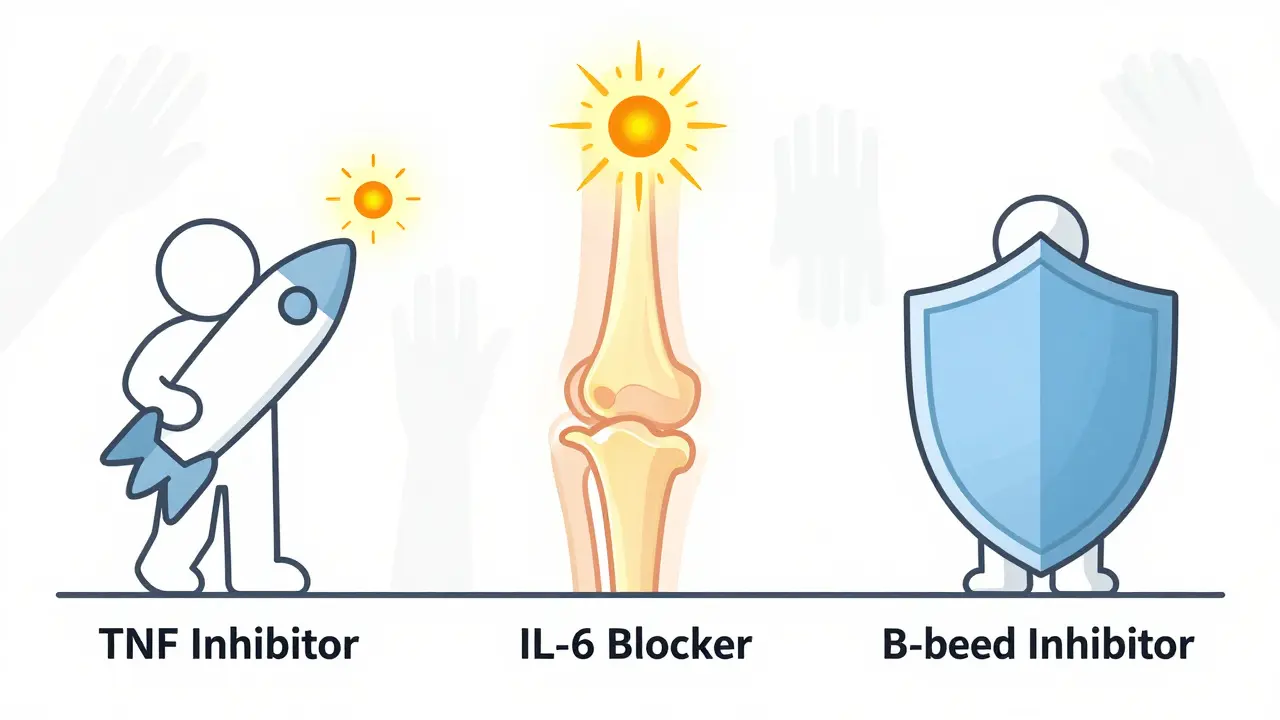
The Major Types of Biologic Therapies
There are four main classes of biologics used for RA today:
- TNF inhibitors (like adalimumab, etanercept, infliximab): These block tumor necrosis factor, a key inflammatory protein. They were the first biologics approved (etanercept in 1998) and still make up 55% of all biologic prescriptions.
- IL-6 inhibitors (like tocilizumab): These stop interleukin-6, another major driver of joint damage and fatigue. Many patients report dramatic improvements in energy and joint swelling.
- B-cell inhibitors (like rituximab): These remove B-cells, the immune cells that produce harmful antibodies. Often used when TNF inhibitors fail.
- T-cell costimulation blockers (like abatacept): These prevent T-cells from activating and triggering inflammation.
Combining a biologic with methotrexate works better than either alone. Clinical trials show that 60% of patients on this combo see at least a 50% drop in disease activity-compared to just 40% on methotrexate alone.
Real Patient Experiences: Successes and Struggles
People’s stories tell the real story of RA treatment.
Sarah K., 42, stopped playing piano after five years of hand deformities from RA. After starting tocilizumab in 2022, she regained enough hand function to play again. Her story is on the Arthritis Foundation’s patient community-over 150,000 people share experiences like hers.
But not every story is uplifting. On Drugs.com, Humira (adalimumab) has a 6.5/10 average rating. Nearly a third of users report painful injection site reactions. One in three patients stop biologics within the first year-not because they don’t work, but because of side effects or cost.
And cost is a huge barrier. Annual treatment can run from $15,000 to $60,000. Even with insurance, out-of-pocket costs can hit $5,000 a year. A 2023 Arthritis Foundation survey found 41% of patients skipped doses or delayed refills because they couldn’t afford it.
Big Risks: Infections, Cancer, and Long-Term Safety
Biologics work by suppressing parts of your immune system. That’s why they’re powerful. But that same suppression makes you more vulnerable.
Studies show biologic users have a 1.5 to 2 times higher risk of serious infections like tuberculosis, pneumonia, or sepsis. That’s why the FDA requires all prescribers to complete special training under its REMS program. Before starting, you must be screened for TB and hepatitis.
There’s also a small but real increased risk of lymphoma and other cancers. The absolute risk is low-about 1 extra case per 1,000 patients per year-but it’s something you and your doctor must weigh carefully.
And not everyone responds. Some patients try three or four biologics before finding one that works. Others never find one. That’s why researchers are now focusing on predicting who will respond to which drug.
What’s New in RA Treatment?
The field is moving fast.
In September 2023, the FDA approved the first biosimilar to adalimumab-adalimumab-adaz. Biosimilars are nearly identical to the original biologic but cost 15-20% less. That’s a big deal for patients struggling with bills.
Also in 2024, upadacitinib (Rinvoq), a JAK inhibitor, got expanded approval for early RA. JAK inhibitors are oral pills, not injections, making them easier for some patients to take. They’re now grabbing 15% of the RA drug market.
Looking ahead, drugs like deucravacitinib (a TYK2 inhibitor) are in late-stage trials. These target different parts of the immune pathway and may work for patients who’ve failed other biologics.
Why Early Treatment Is Non-Negotiable
Dr. Laura Robbins from the Hospital for Special Surgery says it plainly: “The window to prevent joint damage is the first 3 to 6 months after symptoms start.”
Once bone erosion happens, it’s permanent. No drug can rebuild lost cartilage. That’s why experts now follow a “treat-to-target” strategy: set clear goals (like remission or low disease activity), check your progress every 3-6 months with blood tests and exams, and adjust treatment quickly if you’re not improving.
The European League Against Rheumatism (EULAR) recommends adding a biologic within 3-6 months if methotrexate alone isn’t enough. Delaying means more damage, more pain, and less chance of returning to normal life.
Living with RA: Beyond Medication
Medicine helps-but it’s not the whole answer.
Exercise is critical. Just 150 minutes a week of moderate activity-walking, swimming, cycling-can reduce pain and stiffness. Strength training protects joints. Studies show losing 5-10% of body weight cuts disease activity by 20-30% in overweight patients.
Smoking is a major trigger. If you smoke, quitting is the single best thing you can do to slow RA progression.
Support matters too. The CDC’s self-management workshops help patients learn how to track symptoms, manage flares, and communicate with doctors. People who complete them report 20% less pain after six months.
Apps like MyRA help patients log symptoms, medications, and triggers. Over 250,000 people have downloaded it. It’s not magic-but it gives you control.
What’s Next for RA Patients?
The future is brighter than it was 10 years ago. Biosimilars are lowering costs. New drugs are coming. And doctors are getting better at personalizing treatment.
But challenges remain. Rural patients are 30% less likely to get biologics because of limited access to rheumatologists. Insurance hurdles still block many from starting treatment. And the number of Americans with RA is expected to rise from 1.3 million to 1.7 million by 2030.
What you can do now: If you’ve had joint pain and stiffness for more than six weeks, don’t wait. See a rheumatologist. Get tested. Start early. The right treatment can keep you moving, working, and living-without letting RA take over your life.
Is rheumatoid arthritis the same as osteoarthritis?
No. Osteoarthritis is caused by wear and tear on joints over time, usually in older adults. Rheumatoid arthritis is an autoimmune disease where the immune system attacks joint tissue, causing inflammation. RA can occur at any age, affects joints symmetrically, and can damage organs beyond the joints.
Can biologic therapies cure rheumatoid arthritis?
No, biologics don’t cure RA. But they can put the disease into remission-meaning symptoms disappear or become very mild. Many patients stay in remission for years with consistent treatment. Stopping biologics often leads to flare-ups, so most people need to continue them long-term.
How long does it take for biologics to start working?
Most people notice improvement in 4 to 8 weeks, but full benefits can take 3 to 6 months. Some, like TNF inhibitors, may work faster-within 2 to 4 weeks. Others, like rituximab, require multiple infusions over several months. Patience is key, but if there’s no change after 3 months, talk to your doctor about switching.
Are biologics safe during pregnancy?
Some biologics, like certolizumab and etanercept, are considered low-risk during pregnancy and are often continued to prevent flares. Others, like rituximab and abatacept, are usually stopped before conception. Always discuss pregnancy plans with your rheumatologist-uncontrolled RA poses greater risks to pregnancy than most biologics.
What happens if I stop taking my biologic?
Stopping can cause your RA to flare back quickly-sometimes within weeks. You may lose the progress you made and face increased joint damage. In some cases, the drug may not work as well if you restart it later. Never stop without talking to your doctor. If cost or side effects are the issue, ask about biosimilars or patient assistance programs.
Can I still get vaccines while on biologics?
Yes, but timing matters. Live vaccines (like MMR or shingles vaccine) are generally avoided while on biologics because of infection risk. Inactivated vaccines (like flu, pneumonia, or COVID-19 shots) are safe and strongly recommended. Get them before starting biologics if possible, or during a stable phase of your disease.
Managing RA isn’t about finding one magic pill. It’s about building a plan-medication, movement, monitoring, and support-that works for your life. The goal isn’t just to reduce pain. It’s to protect your future so you can keep doing the things you love.


Cheryl Griffith
January 15, 2026 AT 23:22I remember when I first got diagnosed-morning stiffness so bad I couldn’t hold my coffee cup. Took me months to realize it wasn’t just ‘getting older.’ This post nailed it. The part about emotional toll? Yeah. That’s the silent part no one talks about.
swarnima singh
January 17, 2026 AT 22:18biologics r just big pharma’s way of makin’ us pay 40k a yr 4 sumthin’ that should be free… they dont cure, they just make u feel like ur not dyin’ slow enough. capitalism is the real autoimmune disease.
Isabella Reid
January 19, 2026 AT 08:34My mom’s on tocilizumab and she’s back to gardening. Not full energy, but she can hold a trowel again. It’s not perfect, but it’s life-changing. Also, the CDC workshops? Total game changer. Learned how to track flares with a simple notes app. No fancy tech needed.
Jody Fahrenkrug
January 19, 2026 AT 15:06Just wanted to say-thank you for writing this. I’ve been avoiding the rheumatologist for a year because I was scared. This made me book my appointment. No more waiting.
Kasey Summerer
January 20, 2026 AT 12:00So let me get this straight… we inject ourselves with genetically engineered proteins to stop our body from attacking itself… and we call this medicine? 🤦♀️
kanchan tiwari
January 20, 2026 AT 23:13THEY KNOW. THEY KNOW WHAT THEY’RE DOING. Biologics are just the tip of the iceberg. Vaccines, glyphosate, 5G-all of it’s linked. Why do you think they’re so expensive? Because they don’t want you cured. They want you dependent. I read a paper once-
Bobbi-Marie Nova
January 22, 2026 AT 10:05Same. I skipped doses last year because of the copay. Felt like a failure. Then I found a patient assistance program through the Arthritis Foundation. Got my meds for $5/month. No shame in asking for help. You’re not alone.
Allen Davidson
January 23, 2026 AT 12:58Don’t let the fear of side effects stop you. I was scared of infections too-until I got pneumonia on methotrexate alone. Biologic saved my lungs. Yes, you’re more vulnerable. But so is your body without it. Talk to your doc. Don’t guess.
john Mccoskey
January 23, 2026 AT 18:59Let’s be brutally honest: biologics are a band-aid on a bullet wound. You’re not treating RA-you’re suppressing a symptom of systemic immune dysregulation caused by decades of processed food, environmental toxins, and chronic stress. The real solution? Eliminate gluten, fix your gut microbiome, reduce cortisol, and stop treating your body like a machine. But no, the pharmaceutical industry would rather sell you $60K/year vials than admit the root cause is your lifestyle. You’re being sold a lie wrapped in clinical trials and FDA approval.
And don’t even get me started on biosimilars. They’re not ‘nearly identical’-they’re slightly cheaper knockoffs with different impurity profiles. The FDA doesn’t require full bioequivalence testing for them. You’re a guinea pig. And yet, people cheer because it’s ‘affordable.’
Meanwhile, the real breakthroughs-like TYK2 inhibitors-are being buried under patent wars and insurance red tape. The system is broken. And you’re all just arguing about which flavor of suppression to take.
Wake up. The answer isn’t in a syringe. It’s in your kitchen, your sleep schedule, and your ability to say no to corporate medicine.
And if you think exercise is ‘critical’? Congrats. You’ve been told the same thing since 1985. It helps. But it doesn’t reverse bone erosion. Only time, and the right drug, can do that.
Ryan Hutchison
January 24, 2026 AT 20:20Why are we letting foreign companies control our meds? Adalimumab was invented in the U.S. Now it’s made in India and China. We’re paying for our own betrayal. Time to bring biologic production back home and stop outsourcing our health.
Samyak Shertok
January 25, 2026 AT 02:11Oh so now it’s ‘treat-to-target’? Funny how the same doctors who told me ‘it’s just arthritis’ 5 years ago are now preaching early intervention like they discovered fire. Meanwhile, I waited 3 years because my GP said ‘take ibuprofen and stretch.’ Guess what? My joints are toast. And now I’m on the most expensive drug in the world because YOU waited.
Also, JAK inhibitors? Yeah, sure. Just like the ones that got pulled for heart risks. Who’s gonna be first to die on that one? You? Me? The guy who can’t afford the co-pay so he takes half the dose?
Wake up. This isn’t medicine. It’s a lottery.