The Purple Book isn’t a novel you read for fun-it’s the FDA’s official, searchable database that tells pharmacists, doctors, and patients exactly which biological drugs are biosimilars, which ones are interchangeable, and which ones are the original reference products. If you’ve ever wondered why a pharmacist can swap one insulin for another but not another biologic, the Purple Book holds the answer. It’s the go-to resource for figuring out what can be substituted at the pharmacy counter and what can’t-and why.
What Is the Purple Book, Really?
The Purple Book is a public database run by the U.S. Food and Drug Administration. It lists every biological product approved in the U.S., including the original brand-name drugs and their biosimilar and interchangeable versions. Before 2020, the FDA kept two separate lists-one for drugs under the Center for Drug Evaluation and Research (CDER) and another for biologics under the Center for Biologics Evaluation and Research (CBER). That made it confusing. Now, it’s one unified, searchable tool. You can type in a drug name like Humira or Enbrel, and the Purple Book shows you every biosimilar and interchangeable version approved to match it.
Each product card in the database uses color coding: matching colors mean the products are biosimilar or interchangeable with each other. You’ll also see icons showing how the drug is delivered-autoinjector, prefilled syringe, IV bag-and the date it was approved. The database doesn’t just list names. It tells you whether the product was approved under section 351(a) as a reference product, or under 351(k) as a biosimilar or interchangeable product. That’s the key to understanding the difference between the two.
Biosimilar vs. Interchangeable: The Difference That Matters
Not all biosimilars are created equal. A biosimilar is a biological product that’s highly similar to its reference drug-no clinically meaningful differences in safety, purity, or potency. That means it works the same way, has the same side effects, and delivers the same results. But being biosimilar doesn’t mean you can swap it in automatically at the pharmacy.
An interchangeable biosimilar meets all the same standards as a biosimilar, but it goes further. The FDA requires extra studies to prove that switching back and forth between the interchangeable product and the original reference drug won’t increase risk or reduce effectiveness. Imagine a patient on a reference biologic for rheumatoid arthritis. If they switch to an interchangeable biosimilar, then switch back, then switch again-over and over-their body should respond the same way every time. That’s the bar for interchangeability.
The FDA is clear: interchangeability doesn’t mean the drug is better. It just means it’s safe to swap without a doctor’s permission-under the right state laws. As of late 2023, only seven biosimilars had earned this designation. Two are insulins. Three treat inflammatory conditions like Crohn’s or psoriasis. Two are for eye diseases. That’s it. And the number hasn’t jumped dramatically since.
Why Interchangeability Isn’t Just a Federal Decision
Here’s where it gets messy. The FDA gives the green light for interchangeability. But whether a pharmacist can actually substitute the drug without calling the doctor? That’s up to each state.
As of 2023, 47 states and Puerto Rico allow pharmacists to substitute an interchangeable biosimilar for the prescribed reference product without needing to contact the prescriber. But even then, many states require the pharmacist to notify the doctor, document the switch, or inform the patient. Some states require the patient to consent. Others let the pharmacist make the call, but only for new prescriptions-not for patients already on the original drug.
This patchwork of rules makes it hard for patients to know what’s allowed where. A patient in Texas might get a substituted insulin without a second thought. A patient in California might be asked to sign paperwork. A patient in New York might have to wait for the doctor’s approval. The Purple Book tells you what’s federally approved. It doesn’t tell you what your local pharmacy can do.
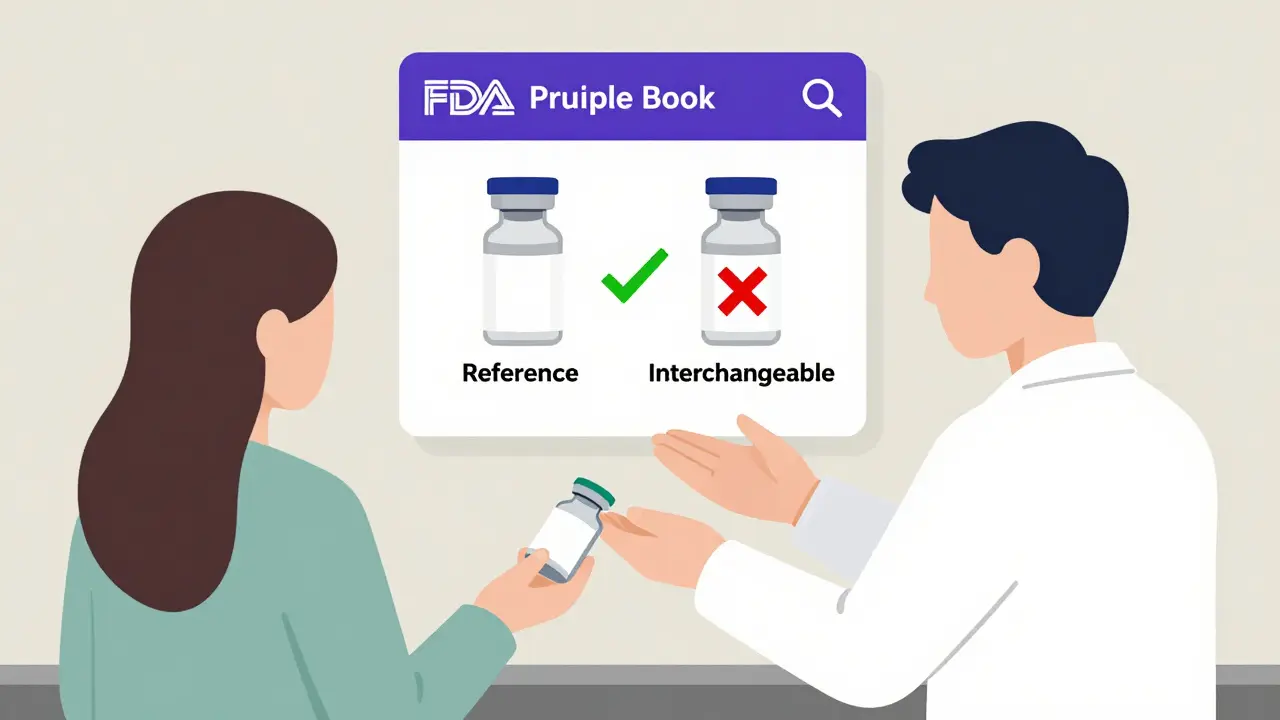
What the Purple Book Doesn’t Tell You
There’s a common misunderstanding: some people think the Purple Book lists “unbranded biologics” as interchangeable. It doesn’t. The FDA says unbranded biologics-drugs that are identical to the reference product but sold without a brand name-are considered equivalent. But they’re not classified as interchangeable biosimilars under the 351(k) pathway. That’s a legal distinction. Only products approved under the biosimilar pathway with the specific interchangeability designation appear in the Purple Book under the 351(k) Interchangeable category.
Also, the Purple Book doesn’t list pricing. It won’t tell you which biosimilar is cheaper. It won’t tell you which one your insurance covers. It’s purely about regulatory status. You still need to check with your pharmacy or insurer for cost and coverage.
How to Use the Purple Book Like a Pro
If you’re a pharmacist, you can search by brand name, generic name, or manufacturer. The results group all biosimilars and interchangeable versions under the reference product. Click on a product card, and you’ll see its approval date, exclusivity status, and whether it’s interchangeable. You can filter by product type-insulin, monoclonal antibodies, vaccines, gene therapies.
If you’re a patient or caregiver, you can look up your prescribed biologic and see if there’s an interchangeable version approved. Then, ask your pharmacist: “Is there an interchangeable biosimilar for this? Can it be substituted here in [your state]?”
Doctors can use it to understand what’s available and what’s legally substitutable. If a patient’s insurance denies coverage for the brand-name drug, the Purple Book helps identify which biosimilar options are actually approved for substitution.
What’s Coming Next?
The FDA is working on clearer labeling rules for biosimilars and interchangeable products. The goal? Make sure prescriptions and pharmacy labels don’t confuse patients. Companies are actively applying for interchangeability status-especially for high-cost drugs like infliximab and adalimumab. More approvals are expected in 2025 and beyond.
But the real bottleneck isn’t science. It’s policy. Until state laws align with federal approvals, the promise of lower-cost, easily substituted biosimilars will be uneven across the country. The Purple Book gives you the facts. It’s up to states, insurers, and providers to turn those facts into real access.
Why This Matters for You
Biologics are expensive. Some cost over $20,000 a year. Biosimilars can cut that cost by 30% to 50%. Interchangeable biosimilars? They have the potential to cut it even further-because they can be swapped without extra paperwork or delays. That means more patients get the treatment they need, faster.
But if you don’t know how to read the Purple Book, you might miss out. You might think all biosimilars are interchangeable. You might assume your pharmacist can switch your drug without asking. You might be wrong. And that could mean delays, confusion, or even higher out-of-pocket costs.
The Purple Book is your free, official source for clarity. Use it. Know what’s approved. Know what’s substitutable. Know what your state allows. That’s how you take control of your care-or your patients’ care.
Is the Purple Book the same as the Orange Book?
No. The Orange Book lists approved generic drugs and their reference brand-name small-molecule drugs. The Purple Book does the same thing-but for biological products, which are much more complex. Biologics are made from living cells, not chemicals, so they need a separate tracking system. The Orange Book is for pills like generic lisinopril. The Purple Book is for injectables like Humira and its biosimilars.
Can a pharmacist substitute a biosimilar without my permission?
Only if it’s an FDA-approved interchangeable biosimilar AND your state allows substitution without prescriber approval. Most states do-but not all. Even then, some require the pharmacist to notify you or your doctor. Always ask your pharmacist if substitution is happening and why.
Are interchangeable biosimilars safer than regular biosimilars?
No. The FDA says interchangeable biosimilars are not safer or more effective. They just have extra data proving that switching between them and the original drug doesn’t cause harm. Both types are equally safe and effective for initial use. Interchangeability is about substitution flexibility, not superiority.
Why are so few biosimilars approved as interchangeable?
Because the clinical studies required are more complex and expensive. To prove interchangeability, companies must run switching studies-where patients alternate between the biosimilar and the reference drug multiple times. These studies take years and cost millions. Many companies choose to skip it and just seek biosimilar approval, which is enough to enter the market. Interchangeability is a bonus, not a requirement.
Where can I find the Purple Book?
Go to the FDA’s official website: fda.gov/drugs/biosimilars/purple-book. It’s free, searchable, and updated monthly. You don’t need to log in or pay anything. Bookmark it. It’s the only official source for this information.
Does the Purple Book include vaccines and gene therapies?
Yes. The Purple Book includes all biological products approved under the Public Health Service Act. That means not just monoclonal antibodies and insulins, but also vaccines, blood products, cellular therapies, and gene therapies. If it’s a biologic and approved by the FDA, it’s in the Purple Book.



laura Drever
January 12, 2026 AT 23:13The purple book is such a mess why does it even exist if no one knows what the hell is interchangeable or not
jefferson fernandes
January 14, 2026 AT 08:57Let’s be real-this is one of the most important public health tools we’ve got, and yet most pharmacists don’t even know how to use it properly. The FDA did their part: clear, searchable, color-coded, updated monthly. But the real failure? The lack of standardized state laws. You can’t have a national system if every state thinks it’s a sovereign nation when it comes to insulin substitution. This isn’t about bureaucracy-it’s about access. Patients are paying thousands more because of legal inertia. Fix the laws, not the database.
James Castner
January 15, 2026 AT 21:42There’s a deeper philosophical question here: if a drug is functionally identical, why does the legal architecture demand such an artificial distinction between ‘biosimilar’ and ‘interchangeable’? We’ve built a regulatory system that treats biological molecules like they’re mechanical parts-when in reality, they’re living systems, produced in complex bioreactors, subject to subtle batch variations even in originators. The FDA’s criteria for interchangeability is a noble attempt to quantify the unquantifiable. But we’re not engineering a gear; we’re managing a biological dance. The fact that we’ve only approved seven interchangeable products after a decade of the BPCIA suggests we’re not ready for the science we’ve unleashed. We need humility, not just more studies.
lucy cooke
January 17, 2026 AT 15:11Oh my god, the Purple Book? I mean, really? This is what we’ve come to? A government database with color-coded icons and a sad little footnote about state laws? It’s like the FDA handed us a Shakespearean sonnet and said ‘read this while your insulin co-pay goes up.’ I’m not even mad-I’m just… profoundly disappointed. We could’ve had flying cars. Instead, we have pharmacists whispering about ‘substitution protocols’ like they’re secret society passwords. This isn’t healthcare. This is performance art.
Clay .Haeber
January 19, 2026 AT 06:51Oh wow, so the Purple Book is just a glorified Excel sheet with a purple background? And you’re telling me we spent $200 million to build this because someone thought ‘Orange Book’ was too mainstream? Brilliant. Next up: the Green Book for antibiotics, the Red Book for opioids, and the Black Book for when your insurance denies everything anyway. Also, ‘interchangeable’? That’s just corporate-speak for ‘we’re too scared to let pharmacists do their job.’
Priyanka Kumari
January 20, 2026 AT 12:09This is such an important resource, and I’m so glad it’s free and public. As a pharmacist in India working with biologics imported to low-resource settings, I can’t tell you how valuable it is to have clear regulatory status. Even though we don’t have interchangeable biosimilars here yet, understanding the FDA’s framework helps us advocate for better policies. Thank you for making this accessible. I’ve shared this with my colleagues-every healthcare worker should know about it.
Vinaypriy Wane
January 22, 2026 AT 05:32Wait-so you’re telling me that even if a biosimilar is FDA-approved as interchangeable, my pharmacist still can’t switch it unless my state says so? And some states require me to sign a form? And others won’t switch me if I’m already on the brand? This is insane. I’ve been on Humira for five years. My co-pay is $500 a month. There’s a cheaper, approved, interchangeable version. But because I live in Ohio, I can’t get it unless my doctor writes a new script? This isn’t healthcare-it’s a bureaucratic obstacle course designed to keep prices high.
Diana Campos Ortiz
January 23, 2026 AT 09:37just found this and wow… i had no idea the purple book even existed. i’ve been paying full price for my biologic for years thinking there were no cheaper options. now i know there’s one approved and interchangeable… but my pharmacist said they can’t switch me unless my doctor approves. i’m gonna call my doc tomorrow. thank you for this.
Jesse Ibarra
January 23, 2026 AT 11:21Oh, so the FDA approved seven interchangeable biosimilars? That’s cute. Meanwhile, Big Pharma is still charging $20K/year for the original drugs while the biosimilars sit on shelves because insurers won’t cover them without prior auth and the states won’t let pharmacists substitute. This isn’t a public health win-it’s a corporate lobbying victory. The Purple Book is a shiny distraction. The real story? The money. Always the money.
Acacia Hendrix
January 25, 2026 AT 10:40Let’s not conflate regulatory pathways with therapeutic equivalence. The Purple Book’s 351(k) designation is a statutory construct, not a clinical endorsement. The FDA’s criteria for interchangeability-extrapolation of data, multiple-switching studies, immunogenicity profiles-are not indicators of superiority, but rather compliance with a specific regulatory taxonomy. To conflate this with clinical efficacy is to misunderstand the entire biologics approval paradigm. This is not a consumer product catalog; it’s a legal instrument embedded in the PHSA.
Adam Rivera
January 25, 2026 AT 14:52Hey, just wanted to say this post was super helpful! I’m from Nigeria and we don’t have a system like this yet, but I’m trying to push for one. Seeing how the U.S. tracks biosimilars gave me a blueprint. I showed this to my med school professor and she’s now helping me draft a proposal. Thanks for breaking it down so clearly. Sometimes the most boring stuff-like a government database-is the most powerful.
sam abas
January 26, 2026 AT 02:06Wait, so the purple book doesn’t list prices? And you’re surprised people are confused? That’s like publishing a car manual that lists every model’s engine specs but not the MSRP. Also, ‘interchangeable’? That’s just a fancy word for ‘we’re letting pharmacists play Russian roulette with your immune system.’ And don’t even get me started on the fact that only 7 are approved-because the studies cost millions, but the brand-name companies are paying lobbyists millions more to block it. This isn’t science-it’s a money laundering scheme with a spreadsheet.