Diabetes Foot Care: Ulcer Prevention and Daily Inspection Checklist
- Dorian Wakefield
- 5 Jan 2026
- Health and Wellness
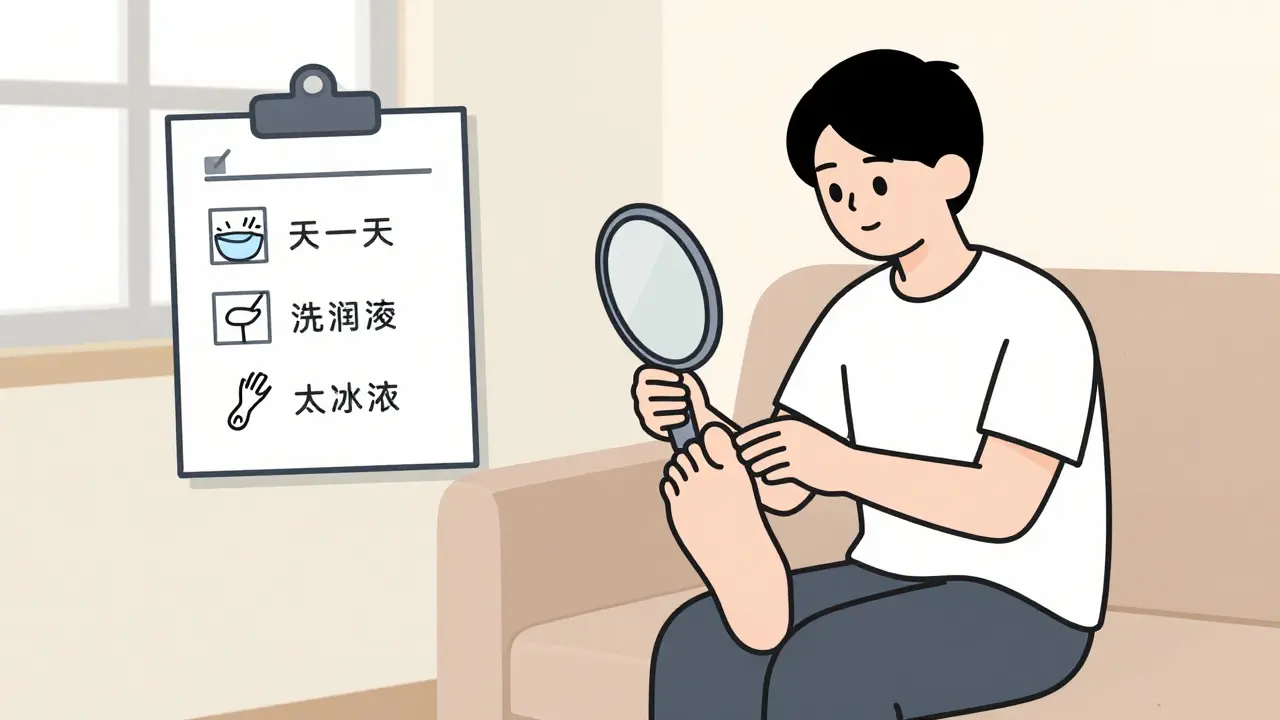
Learn how to prevent diabetic foot ulcers with a daily inspection checklist, proper footwear tips, and what to watch for. Most ulcers are avoidable with consistent care.
View More