Grapefruit and Grapefruit Juice: Which Medications Are Affected and Why
- Dorian Wakefield
- 31 Jan 2026
- Health and Wellness
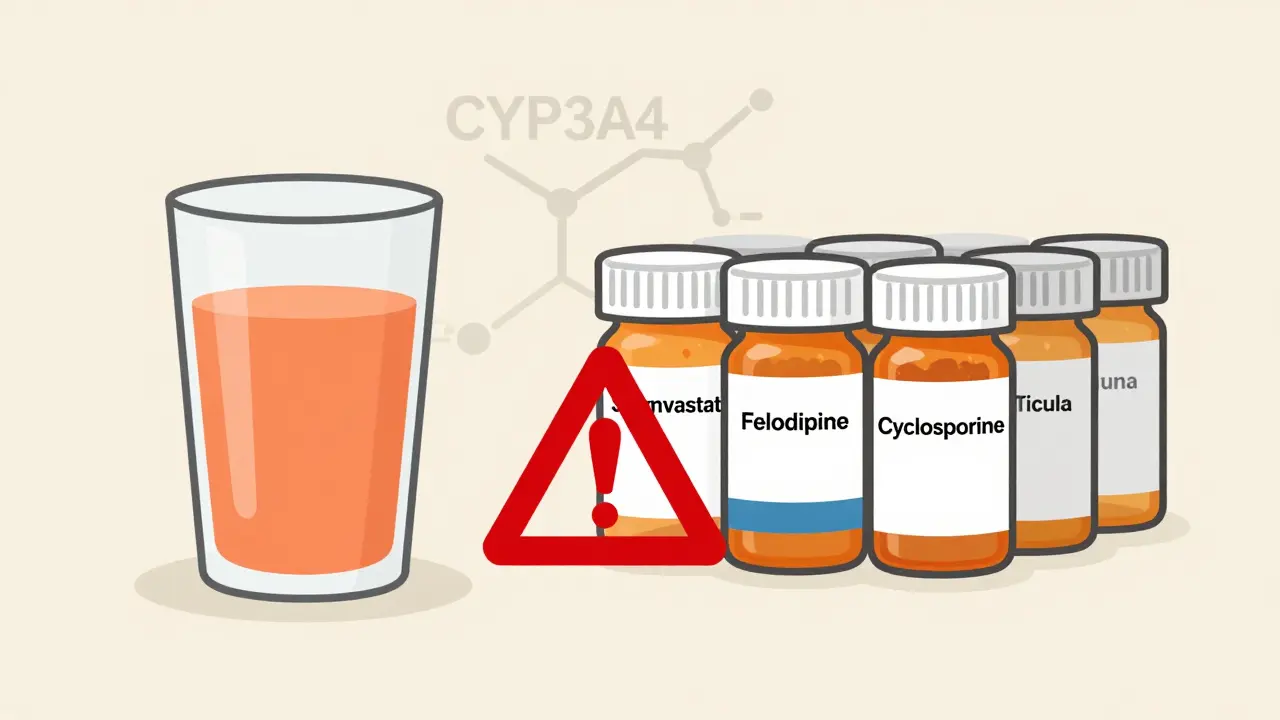
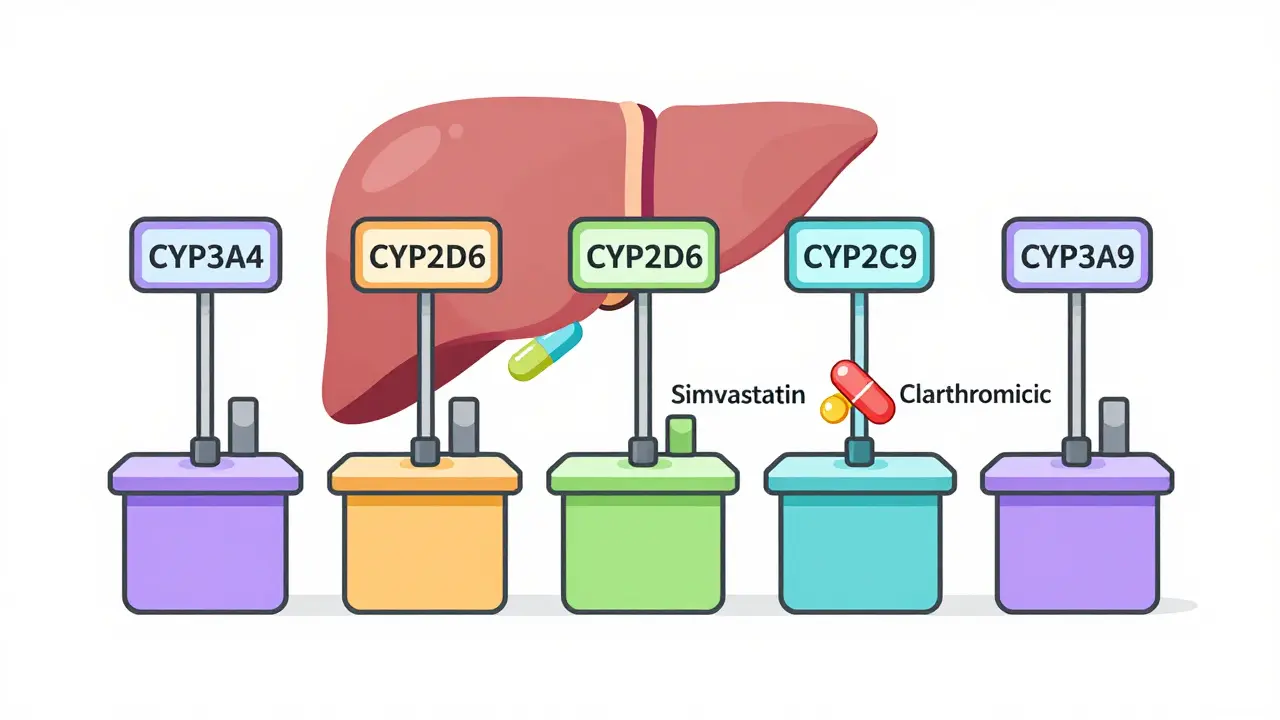
Grapefruit can dangerously increase levels of many medications by blocking enzymes that break them down. Statins, blood pressure drugs, and immunosuppressants are among the most affected. Avoid grapefruit entirely if you're on these meds.
View More